Your Complete Guide to Different Types and How They Work
Think of your body as a massive construction project that never really ends. Every day, millions of your cells wear out, get damaged, or simply reach the end of their useful life. Your skin cells shed constantly, your blood cells have a lifespan of about 120 days, and even your bone cells are continuously being broken down and rebuilt. Yet somehow, your body keeps functioning perfectly, replacing what's lost with fresh, healthy cells. The secret behind this remarkable repair system? Stem cells.
These extraordinary cells are like the ultimate Swiss Army knife of biology. While most cells in your body have a specific job – heart cells pump blood, nerve cells transmit signals, muscle cells contract – stem cells have kept their options open. They're the undecided college freshmen of the cellular world, capable of becoming almost anything they want to be when they grow up.
But here's what makes stem cells truly special: they don't just turn into other types of cells, they can also make copies of themselves. Imagine having a magical tool that could not only transform into any other tool you needed but could also create more copies of itself. That's essentially what stem cells do, and it's why scientists and doctors are so excited about their potential for treating diseases and injuries that were once considered incurable.
The Master Cells That Keep You Alive
Every single cell in your body can trace its ancestry back to stem cells. When you were just a tiny cluster of cells developing in your mother's womb, you were essentially made entirely of stem cells. These early stem cells had an incredible responsibility: they needed to create every type of cell that would eventually make up your entire body.
Your body contains roughly 37 trillion cells, and they come in about 200 different varieties. You have nerve cells that can stretch several feet long, tiny red blood cells that squeeze through the smallest capillaries, muscle cells that can contract with tremendous force, and bone cells that create structures stronger than concrete. The fact that all of these incredibly diverse cell types originated from stem cells is nothing short of miraculous.
What makes stem cells so versatile is their unique ability to remain in what scientists call an "undifferentiated" state. Think of differentiation as choosing a career path. Once a cell differentiates into a heart cell, it's committed to that job for life. It can't suddenly decide to become a brain cell instead. But stem cells are like permanent career counselors – they can guide other cells toward any profession while maintaining their own flexibility to choose different paths as needed.
Adult Stem Cells: Your Body's Repair Crew
You might think that stem cells are only important during development, but that couldn't be further from the truth. Right now, as you're reading this, adult stem cells throughout your body are hard at work maintaining and repairing your tissues.

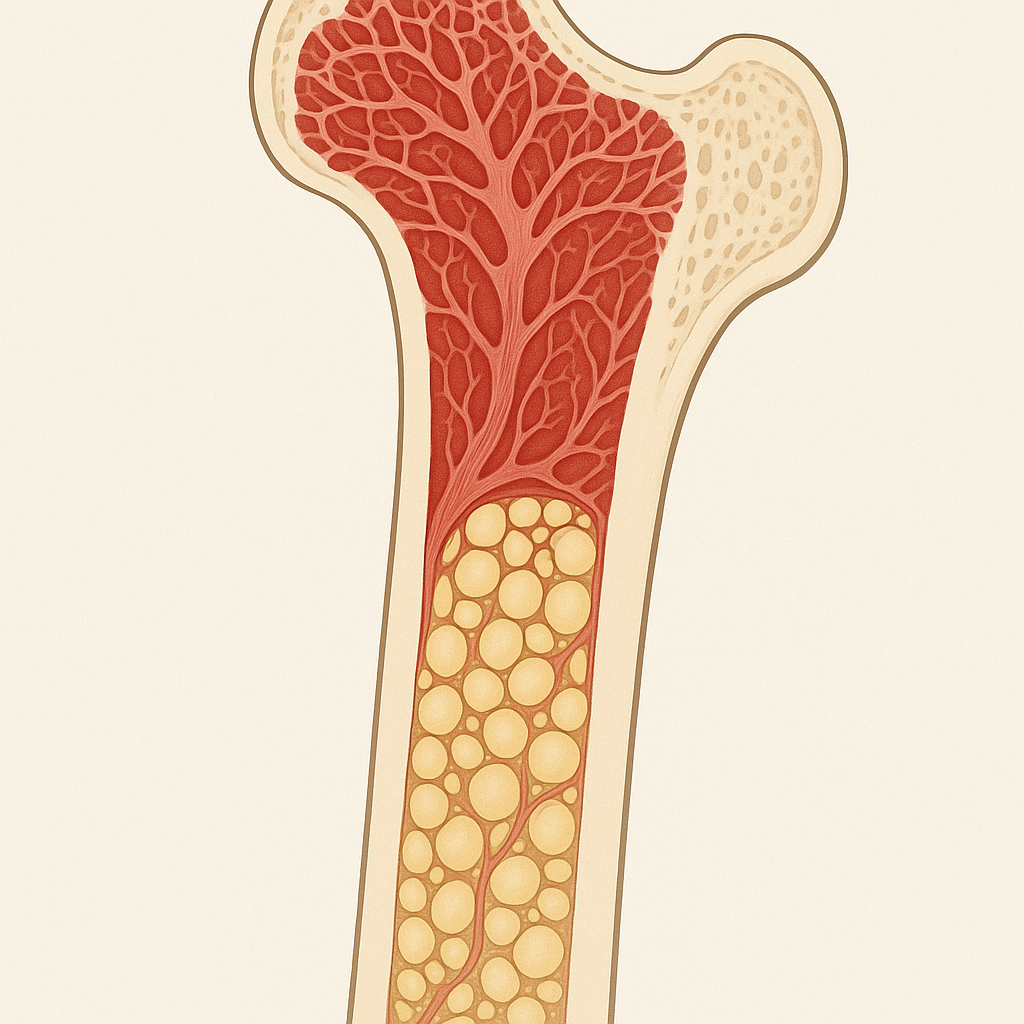
Your bone marrow is like a bustling factory that never closes, churning out millions of new blood cells every day. The stem cells living there are called hematopoietic stem cells, and they're responsible for creating every type of blood cell your body needs: red blood cells to carry oxygen, white blood cells to fight infections, and platelets to help your blood clot when you get injured.
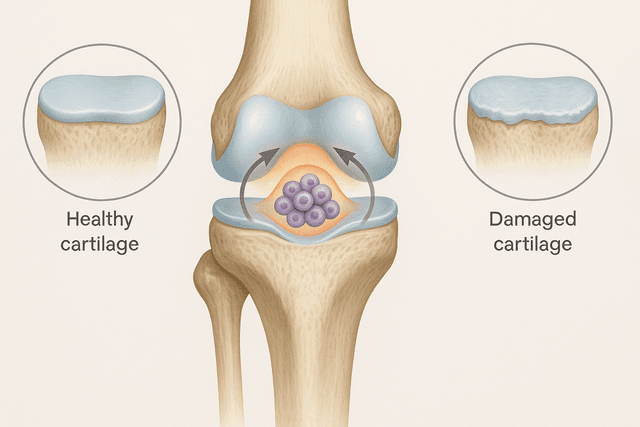
But bone marrow isn't the only place where adult stem cells hang out. Your fat tissue contains mesenchymal stem cells that can turn into bone, cartilage, muscle, or fat cells as needed. Your brain has neural stem cells that can create new neurons and supporting brain cells. Your intestines have some of the most active stem cells in your body, completely replacing the lining of your gut every few days.
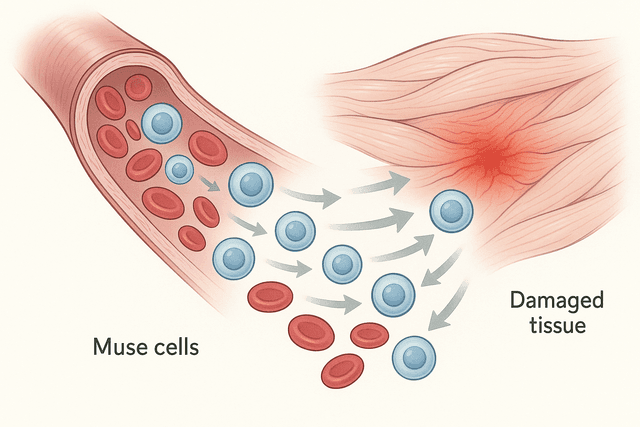
The amazing thing about adult stem cells is how they know when and where they're needed. When you cut your finger, stem cells in your skin receive chemical signals that tell them to start producing new skin cells to close the wound. When you exercise and stress your muscles, stem cells begin creating new muscle fibers to make you stronger. It's like having a perfectly coordinated repair crew that knows exactly what needs to be fixed and how to fix it.
Adult stem cells are also incredibly specialized for their environments. The stem cells in your eyes are different from those in your liver, which are different from those in your muscles. This specialization makes them extremely good at maintaining their specific tissues, but it also limits their flexibility. A stem cell from your muscle tissue can create different types of muscle and connective tissue cells, but it can't suddenly start making brain cells.
Embryonic Stem Cells: The Ultimate Multitaskers
If adult stem cells are like skilled specialists, embryonic stem cells are like universal geniuses. These remarkable cells come from very early embryos, just a few days after fertilization, when the developing organism is still just a hollow ball of cells called a blastocyst.
What makes embryonic stem cells so special is their pluripotency – their ability to become absolutely any type of cell in the human body. While adult stem cells are somewhat restricted in what they can become, embryonic stem cells have unlimited potential. Give them the right chemical signals, and they can become heart cells, brain cells, liver cells, or any of the other 200-plus cell types in your body.
This incredible versatility makes embryonic stem cells extremely valuable for research and potentially for treating diseases. Scientists can grow them in laboratory dishes and study exactly how cells develop and differentiate. They can test new drugs on different types of cells created from embryonic stem cells. And theoretically, they could use these cells to replace any damaged or diseased tissue in the human body.
However, embryonic stem cells also come with significant challenges. Because they come from embryos, their use raises important ethical questions that different people answer differently. Additionally, these cells are so powerful that they can sometimes form tumors if not carefully controlled. Scientists have spent decades learning how to direct embryonic stem cells toward becoming specific cell types without causing unwanted side effects.
Induced Pluripotent Stem Cells: The Game Changers
In 2006, a Japanese scientist named Shinya Yamanaka made a discovery that would revolutionize stem cell research and earn him a Nobel Prize. He figured out how to take ordinary adult cells – like skin cells – and reprogram them to become pluripotent stem cells, essentially turning back their biological clock.
These induced pluripotent stem cells, or iPSCs, are created by introducing four specific genes into adult cells. These genes act like a biological time machine, erasing the cell's memory of what it used to be and returning it to an embryonic-like state where it can become any type of cell again.
The creation of iPSCs solved many of the problems associated with embryonic stem cell research. Since iPSCs can be made from a patient's own cells, there are no ethical concerns about using embryos. Even better, because they come from the patient's own body, there's no risk of immune rejection if they're used for treatment.
iPSCs have opened up entirely new possibilities for personalized medicine. Doctors could theoretically take some of your skin cells, reprogram them into iPSCs, then direct those iPSCs to become whatever type of cell you need to treat your condition. Need new heart cells after a heart attack? Your iPSCs could become heart cells. Need new neurons to treat Parkinson's disease? Your iPSCs could become dopamine-producing brain cells.
Scientists are also using iPSCs to create "disease in a dish" models. By taking cells from patients with genetic diseases and reprogramming them into iPSCs, researchers can create laboratory models of those diseases to study how they develop and test potential treatments. This approach has already led to new insights into conditions like ALS, Huntington's disease, and various forms of heart disease.
How Stem Cells Actually Work: The Science Behind the Magic
Understanding what stem cells can do is fascinating, but understanding how they do it is even more remarkable. The process by which a stem cell decides what type of cell to become involves an incredibly complex dance of genes, proteins, and chemical signals.

Think of each stem cell as containing a massive library of instruction manuals – one manual for how to become a heart cell, another for how to become a brain cell, another for how to become a liver cell, and so on. In any given stem cell, most of these manuals are kept locked away in storage, inaccessible and unused. The process of differentiation involves opening the right instruction manual while keeping all the others locked up.
This process is controlled by transcription factors – special proteins that can turn genes on or off. When a stem cell receives the right combination of chemical signals from its environment, specific transcription factors become active and begin reading from particular instruction manuals. Once this process starts, it tends to be irreversible. The cell begins producing the proteins characteristic of its new identity and stops producing the proteins that kept it in a stem cell state.
The environment plays a crucial role in determining what a stem cell becomes. In your bone marrow, chemical signals promote the formation of blood cells. In your muscles, different signals encourage the creation of muscle cells. Scientists have learned to manipulate these environmental signals in laboratory settings, allowing them to direct stem cells to become whatever type of cell they want to study or use for treatment.
One of the most fascinating aspects of stem cell biology is how these cells balance their two main functions: self-renewal and differentiation. When a stem cell divides, it can produce two new stem cells (self-renewal), two differentiated cells (differentiation), or one of each (asymmetric division). This balance is crucial for maintaining healthy tissues throughout your lifetime.
The Stem Cell Hierarchy: From Totipotent to Committed
Not all stem cells are created equal. Scientists classify stem cells based on their potency – their ability to become different types of cells. Understanding this hierarchy helps explain why different types of stem cells are useful for different purposes.
At the top of the hierarchy are totipotent stem cells. These are the ultimate stem cells, capable of creating not just every type of cell in your body, but also the supporting structures needed for development, like the placenta. In humans, only the fertilized egg and the cells created during the first few divisions after fertilization are truly totipotent.
Next come pluripotent stem cells, which include embryonic stem cells and iPSCs. These cells can become any type of cell in your body, but they can't create the supporting structures needed for development. This makes them incredibly valuable for research and potential therapies, but it also means they need to be carefully controlled to prevent them from forming tumors.
Below pluripotent stem cells are multipotent stem cells, which include most adult stem cells. These cells are more limited in what they can become, but they're also more stable and less likely to cause problems. The hematopoietic stem cells in your bone marrow are multipotent – they can become any type of blood cell, but they can't become liver cells or brain cells.
At the bottom of the hierarchy are oligopotent and unipotent stem cells. Oligopotent stem cells can become a few different but closely related cell types. Unipotent stem cells can essentially only make more of themselves, though they still play important roles in tissue maintenance and repair.
Stem Cells in Action: Your Body's Daily Maintenance
To really appreciate how remarkable stem cells are, consider what they accomplish in your body every single day. Your skin completely replaces itself every 2-3 weeks, which means stem cells in your skin are constantly dividing and differentiating to create new skin cells. The lining of your small intestine replaces itself every 2-3 days, making it one of the most active stem cell environments in your body.
Your blood is perhaps the best example of stem cells in action. Every second, your body produces about 2.5 million new red blood cells. Over the course of a day, that adds up to more than 200 billion new red blood cells, all created by hematopoietic stem cells in your bone marrow. These same stem cells also produce the white blood cells that fight infections and the platelets that help your blood clot.
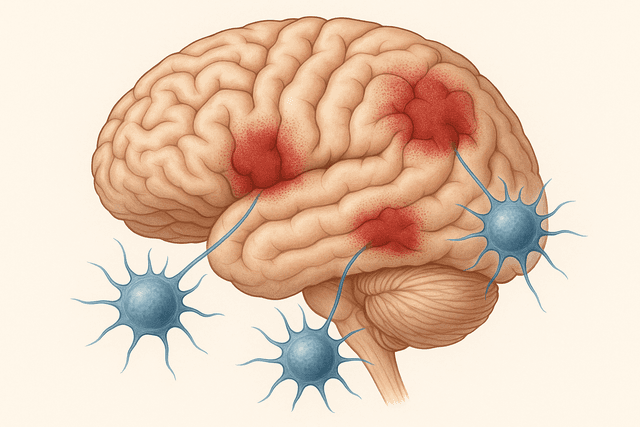
Even tissues that don't regenerate as quickly still depend on stem cells for maintenance and repair. Your muscles contain satellite cells that normally remain quiet but spring into action when you exercise or get injured. Your liver has stem cells that can rapidly multiply to replace damaged tissue. Your brain has neural stem cells that continue creating new neurons throughout your life, particularly in areas involved in learning and memory.
The Challenges of Working with Stem Cells
Despite their incredible potential, working with stem cells isn't easy. Each type of stem cell has its own challenges and limitations that scientists and doctors must overcome.
Adult stem cells are relatively safe and well-understood, but they're also limited in what they can do. They tend to lose their regenerative ability as we age, and they can't be easily expanded in large numbers in laboratory settings. Getting enough adult stem cells for therapeutic use often requires invasive procedures, and the cells don't always work as well outside their natural environment.
Embryonic stem cells and iPSCs are much more versatile, but they're also much more difficult to control. Because they can become any type of cell, they have a tendency to form tumors called teratomas if they're not properly directed. Scientists have spent years learning how to guide these cells toward becoming specific cell types while preventing unwanted differentiation.
There's also the challenge of integration. Even if you can create the right type of cells from stem cells, getting them to properly integrate into existing tissues and function normally is extremely difficult. The cells need to connect with surrounding cells, receive the right chemical signals, and coordinate their activity with the rest of the tissue.
Quality Control: How Your Body Keeps Stem Cells in Check
Your body has evolved sophisticated mechanisms to keep stem cells functioning properly and prevent them from causing problems. Stem cells are constantly monitored by various quality control systems that ensure they divide at the right time, differentiate when appropriate, and eliminate themselves if they become damaged or potentially dangerous.
One of the most important quality control mechanisms is the DNA damage response. Because stem cells need to maintain their genetic integrity over long periods, they have enhanced systems for detecting and repairing DNA damage. If the damage is too severe to repair, these systems trigger the stem cell to destroy itself rather than risk passing on mutations to its daughter cells.
Stem cells also have enhanced mechanisms for dealing with metabolic stress and reactive oxygen species – harmful molecules that can damage cells. This is particularly important because stem cells often live in low-oxygen environments and need to maintain their function over many years or even decades.
The immune system also plays a role in stem cell quality control. Immune cells can recognize and eliminate stem cells that have become abnormal or potentially cancerous. This immune surveillance is one reason why stem cell therapies need to be carefully designed to avoid triggering unwanted immune responses.
The Future of Stem Cell Understanding
Our understanding of stem cells continues to evolve rapidly. New technologies are allowing scientists to study individual stem cells in unprecedented detail, revealing the incredible complexity of how these cells make decisions about when to divide and what to become.
Single-cell sequencing technologies can now analyze the gene expression patterns of thousands of individual stem cells, revealing previously unknown subtypes and states. Advanced microscopy techniques allow researchers to watch stem cells in action in living tissues. Sophisticated computer models are helping scientists understand the complex networks of genes and proteins that control stem cell behavior.
Perhaps most importantly, scientists are beginning to understand how stem cells change with age and disease. This knowledge is crucial for developing effective stem cell therapies and for understanding how to maintain healthy stem cell populations throughout life.
The field of epigenetics – the study of how gene expression is controlled without changing DNA sequences – has also revolutionized our understanding of stem cells. Scientists now know that stem cells use complex epigenetic mechanisms to maintain their identity and respond to environmental signals. This knowledge is leading to new ways of manipulating stem cells for therapeutic purposes.
Stem Cells and Aging: The Connection You Should Know About
One of the most important discoveries in recent stem cell research is how closely stem cell function is linked to aging. As we get older, our stem cells gradually lose their ability to maintain and repair tissues effectively. This decline in stem cell function is now recognized as one of the fundamental causes of aging.
The stem cells in your muscles, for example, become less active as you age, which contributes to the loss of muscle mass and strength that occurs with aging. The stem cells in your brain also become less active, which may contribute to cognitive decline. Even your hematopoietic stem cells in your bone marrow become less effective over time, which is why older adults are more susceptible to infections and take longer to recover from illness.
Understanding this connection between stem cells and aging has opened up new possibilities for healthy aging interventions. Researchers are exploring ways to rejuvenate aging stem cells, potentially allowing people to maintain better health and function as they get older.
What This Means for You
As a patient or someone interested in stem cell therapies, understanding these basics helps you make informed decisions about potential treatments. Not all stem cell therapies are created equal, and the type of stem cells used can dramatically affect both the potential benefits and risks of treatment.
Therapies using adult stem cells, particularly those that use your own cells, tend to be safer but more limited in their applications. Treatments using more potent stem cells like iPSCs have greater potential but also come with increased risks and complexity.
The field of stem cell therapy is advancing rapidly, with new treatments being developed and tested constantly. By understanding the fundamental biology of stem cells, you're better equipped to evaluate new treatments as they become available and to have informed discussions with your healthcare providers about whether stem cell therapies might be appropriate for your condition.
Remember that stem cells aren't magic bullets that can cure every disease or reverse all damage. They're powerful biological tools that, when used appropriately, can help your body's natural repair processes work more effectively. The key is understanding how they work, what they can and can't do, and how to use them safely and effectively.
The story of stem cells is ultimately the story of how life maintains and renews itself. These remarkable cells represent one of biology's most elegant solutions to the challenge of keeping complex organisms healthy and functional throughout their lives. As our understanding of stem cells continues to grow, so too does our ability to harness their power to treat disease and improve human health.