Imagine if your body had a special type of repair cell that could travel anywhere damage occurred, automatically know what type of tissue needed fixing, and then transform into exactly the right kind of cell to make repairs – all without causing immune reactions or requiring complex matching procedures. It sounds like science fiction, but these remarkable cells actually exist. They're called MUSE cells, and they're already helping patients around the world recover from conditions that were once considered untreatable.
MUSE stands for Multi-lineage differentiating Stress Enduring cells, but you can think of them as nature's ultimate repair specialists. Unlike other stem cells that need careful handling and precise conditions to work properly, MUSE cells are incredibly resilient survivors that can navigate the hostile environment of damaged tissue and get to work immediately.
What makes MUSE cells truly revolutionary isn't just what they can do – it's how they do it. These cells have solved many of the biggest challenges that have limited other stem cell therapies. They don't require tissue matching between donors and recipients. They don't need immunosuppressive drugs to prevent rejection. They don't form tumors. And perhaps most remarkably, they seem to have an almost supernatural ability to find their way to exactly where they're needed most in the body.
The Discovery That Changed Everything
The story of MUSE cells begins with a Japanese researcher named Mari Dezawa, who was studying what happened to stem cells when they were put under extreme stress. In 2010, while working at Tohoku University, she made a discovery that would fundamentally change how we think about cellular repair.
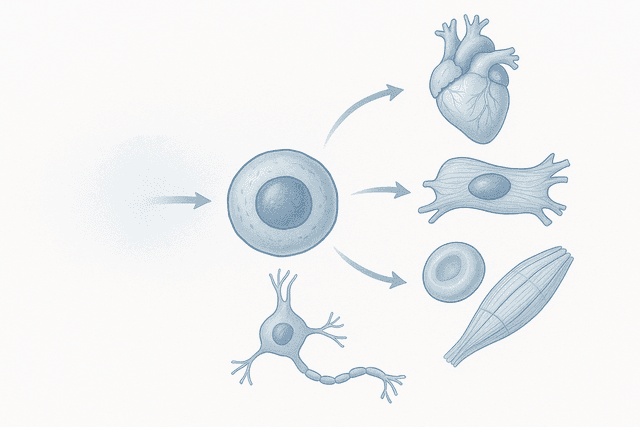
Dezawa found that when certain stem cells were subjected to severe stress – the kind that would kill most cells – a small population not only survived but actually became more powerful. These survivor cells could withstand conditions that would be lethal to ordinary stem cells, and they gained the ability to differentiate into cells from all three primary tissue types in the body: muscle and bone (mesoderm), skin and nervous system (ectoderm), and internal organs (endoderm).
What Dezawa had discovered was essentially a new category of stem cell – one that combined the survival instincts of the toughest cells in the body with the versatility of embryonic stem cells, but without the associated risks and ethical concerns.
Even more intriguing was how these cells behaved when injected into animals with injuries. Unlike other stem cells that might scatter randomly throughout the body, MUSE cells demonstrated an uncanny ability to migrate specifically to sites of damage. It was as if they had built-in GPS systems programmed to locate and repair whatever was broken.
How MUSE Cells Actually Work
The way MUSE cells function is fundamentally different from other stem cell therapies, and understanding this difference helps explain why they're so promising for treating a wide range of conditions.
When most stem cells are introduced into the body, they face several major challenges. First, they need to survive in what is often a hostile environment filled with inflammation and tissue damage. Second, they need to receive the right signals to differentiate into the appropriate cell types. Third, they need to integrate properly with existing tissues. Many stem cell therapies fail because the cells can't overcome one or more of these hurdles.
MUSE cells approach these challenges differently. Their stress-enduring nature means they're already equipped to survive in damaged tissue environments. But more importantly, they seem to respond directly to the molecular distress signals that damaged tissues emit.
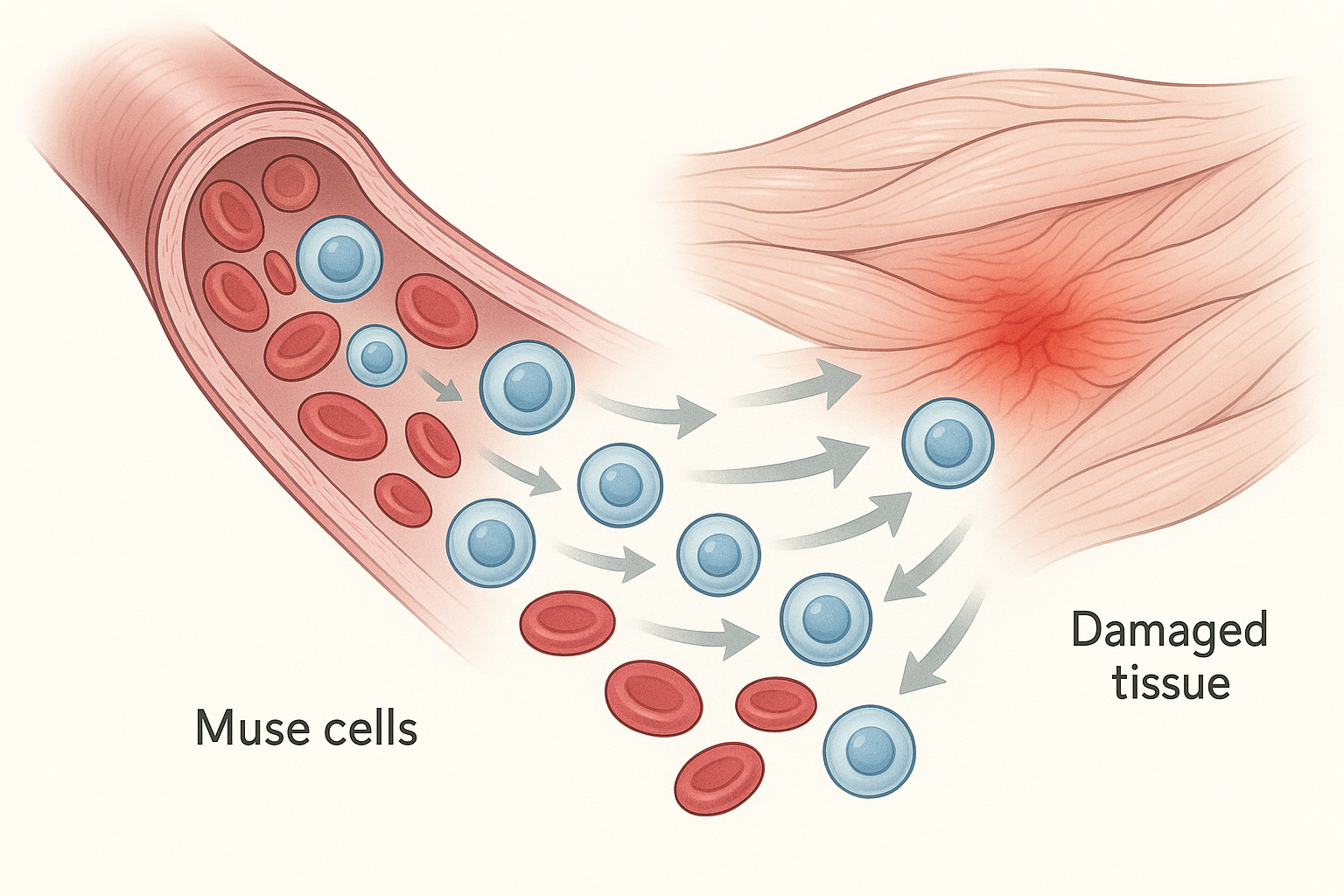
When tissues are injured, they release specific proteins and chemical signals that essentially cry out for help. MUSE cells can detect these signals from a distance and migrate toward them through the bloodstream. Once they arrive at the site of damage, the same signals that attracted them also provide instructions for what type of cells they should become.
This means that MUSE cells don't need to be programmed or directed by doctors – they're self-guided repair units that can assess what's needed and respond appropriately. If they encounter damaged heart tissue, they begin differentiating into heart cells. If they find injured neurons, they start becoming nerve cells. If they discover damaged liver tissue, they transform into liver cells.
Perhaps most remarkably, MUSE cells seem to know when their job is done. Once they've helped repair the damage, they either integrate permanently into the healthy tissue or quietly disappear, preventing the overgrowth that can cause problems with other stem cell therapies.
The No-Matching-Required Advantage
One of the biggest obstacles to widespread stem cell therapy has been the need for tissue matching. Just like organ transplants, most stem cell treatments require careful matching between donors and recipients to prevent immune rejection. This matching process is complex, expensive, and often impossible to achieve perfectly.
MUSE cells have solved this problem in an elegant way. These cells naturally express very low levels of the proteins that trigger immune rejection, and they also secrete substances that actually calm down immune responses rather than provoke them. This means that MUSE cells from one person can be safely given to another person without the need for tissue matching or immunosuppressive drugs.
This characteristic, called immune privilege, is similar to what we see in certain parts of the body like the eyes and brain, where the immune system is naturally less aggressive to prevent damage to critical tissues. MUSE cells seem to carry this immune privilege with them wherever they go, creating a protective bubble that allows them to work without interference from the recipient's immune system.
The implications of this are enormous. It means that MUSE cells can be prepared in advance, stored in cell banks, and made available for immediate use whenever patients need them. There's no waiting for matching, no delay while donors are found, and no need for patients to take immune-suppressing drugs that can leave them vulnerable to infections and other complications.
Real Patients, Real Results
While MUSE cell therapy might sound futuristic, it's already helping real patients in clinical trials around the world. In Japan, where much of the pioneering research has been conducted, patients with conditions ranging from spinal cord injuries to ALS (Lou Gehrig's disease) have been receiving MUSE cell treatments with encouraging results.

One of the most dramatic applications has been in treating spinal cord injuries. Traditionally, these injuries have been considered largely untreatable because the spinal cord has very limited ability to repair itself. However, in clinical trials, patients who received MUSE cell injections within weeks of their injuries showed significant improvements in both sensation and movement.
The treatment process is remarkably simple. Patients receive a single intravenous injection of MUSE cells, similar to getting any other IV medication. The cells then travel through the bloodstream, locate the damaged spinal cord tissue, and begin the repair process. Over the following months, many patients have experienced gradual return of function that doctors previously thought was impossible.
In one notable case, a patient who was completely paralyzed below the chest began regaining sensation and movement in his legs several months after MUSE cell treatment. While he hasn't achieved complete recovery, the improvement has been life-changing, allowing him to stand with assistance and regain some independence.
Similar encouraging results have been seen in patients with ALS, a devastating disease that progressively destroys the neurons that control muscle movement. While MUSE cells haven't cured ALS, patients in clinical trials have shown slower disease progression and, in some cases, temporary improvements in muscle function.
Stroke Recovery: Rewiring the Brain
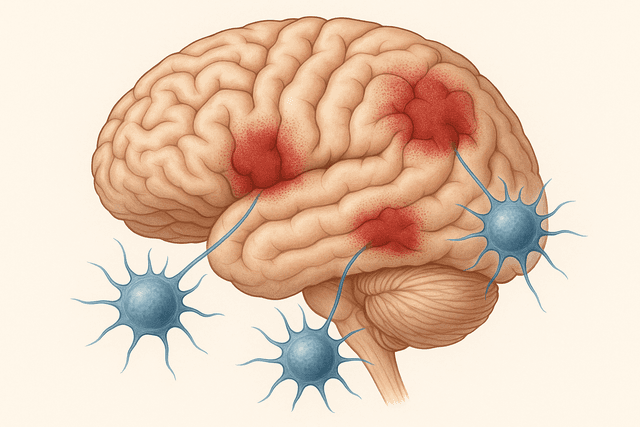
Perhaps nowhere is the potential of MUSE cells more evident than in treating stroke. When someone has a stroke, brain cells die from lack of oxygen, often leaving permanent disabilities like paralysis, speech problems, or cognitive difficulties. Traditional treatments focus on preventing further damage, but there's been little that could actually repair the brain tissue that was lost.
MUSE cells offer a different approach. When injected into stroke patients, these cells can cross the blood-brain barrier – a protective barrier that normally prevents most substances from entering the brain – and migrate to areas of stroke damage. Once there, they can differentiate into various types of brain cells, including neurons and the supporting cells that help neurons function.
In clinical trials, stroke patients who received MUSE cell therapy have shown improvements in areas where recovery was previously thought impossible. Patients who couldn't speak have regained language abilities. Those who couldn't move their arms or legs have recovered mobility. While not all patients improve, and the degree of improvement varies, the results suggest that the brain's capacity for healing may be far greater than previously believed when given the right cellular support.
What's particularly encouraging is that MUSE cells seem to work even when given months or years after a stroke, during periods when conventional wisdom says no further recovery is possible. This suggests that the brain retains the capacity for repair much longer than we thought, but it needs the right cellular tools to activate that repair process.
Beyond the Nervous System: Treating Heart Disease
MUSE cells aren't limited to treating neurological conditions. Their ability to differentiate into multiple cell types makes them potentially valuable for treating a wide range of diseases, including heart disease.
When someone has a heart attack, part of the heart muscle dies from lack of oxygen. The heart tries to compensate by making the remaining muscle work harder, but this often leads to heart failure over time. Traditional treatments focus on managing symptoms and slowing progression, but they can't replace the heart muscle that was lost.
MUSE cells offer the possibility of actually regenerating damaged heart tissue. In animal studies, MUSE cells injected after heart attacks have successfully differentiated into heart muscle cells, blood vessel cells, and the other specialized cells that make up healthy heart tissue. This has led to improved heart function and better survival rates.
Early human trials are now underway to test whether MUSE cells can provide similar benefits for people who have had heart attacks. While results are still preliminary, the animal data suggests that MUSE cells could potentially transform how we treat heart disease, shifting from managing decline to actually promoting recovery.
The Manufacturing Marvel
One of the most impressive aspects of MUSE cell therapy is how these cells are produced and prepared for clinical use. Unlike other stem cell therapies that require complex and expensive manufacturing processes, MUSE cells can be relatively easily isolated and expanded from bone marrow.
The process begins with bone marrow from healthy donors. The bone marrow cells are subjected to a carefully controlled stress protocol that eliminates most cells while allowing the MUSE cells to survive and proliferate. The surviving MUSE cells are then grown in specialized culture conditions that maintain their unique properties while allowing them to multiply.
What makes this process particularly elegant is its efficiency. While other stem cell therapies might require weeks or months to prepare enough cells for treatment, MUSE cells can be prepared relatively quickly and in large quantities. This means that treatments can be made available more readily and at lower costs than many other stem cell therapies.
The cells can also be cryopreserved – frozen in liquid nitrogen – for long-term storage without losing their therapeutic properties. This allows for the creation of cell banks where MUSE cells can be prepared in advance and stored until needed, making treatments available on-demand rather than requiring weeks or months of preparation time.
Global Expansion and Regulatory Progress
While much of the early research on MUSE cells was conducted in Japan, the therapy is now being tested and developed worldwide. Clinical trials are underway or planned in the United States, Europe, and other countries, testing MUSE cells for a variety of conditions.
The regulatory landscape for MUSE cells has been generally favorable, partly because these cells have such a strong safety profile. Unlike embryonic stem cells, MUSE cells don't raise ethical concerns. Unlike gene-modified cells, they don't require genetic manipulation. And unlike many other stem cell therapies, they don't require immunosuppressive drugs or complex matching procedures.
In Japan, MUSE cell therapy has received conditional approval for certain conditions under the country's regenerative medicine framework, allowing patients to access the treatment while longer-term studies continue. This conditional approval pathway, which balances patient access with safety monitoring, may serve as a model for other countries considering how to regulate MUSE cell therapies.
The United States Food and Drug Administration (FDA) has granted several MUSE cell therapies "orphan drug" status for rare diseases, which provides incentives for continued development and may accelerate the approval process. Similar designations are being pursued in Europe and other regions.
What Patients Should Know
For patients considering MUSE cell therapy, there are several important factors to understand. First, while the results from clinical trials have been encouraging, MUSE cell therapy is still an experimental treatment for most conditions. The therapy has been tested in relatively small numbers of patients, and longer-term follow-up data is still being collected.
Second, like any medical treatment, MUSE cell therapy does carry some risks. While serious side effects have been rare in clinical trials, patients have experienced temporary symptoms like fatigue, mild fever, or inflammation at injection sites. More serious complications are possible but appear to be uncommon.
Third, MUSE cell therapy is not a cure-all. While some patients have experienced dramatic improvements, others have seen modest benefits or no improvement at all. The factors that determine who will respond well to treatment are still being studied, making it difficult to predict outcomes for individual patients.
Cost is another important consideration. MUSE cell therapies are currently expensive, and most are not yet covered by insurance. Patients interested in treatment may need to pay out-of-pocket or participate in clinical trials where the treatment is provided at no cost.
The Science Behind the Success
What makes MUSE cells so effective compared to other stem cell therapies? The answer lies in several unique biological properties that scientists are still working to fully understand.
First, MUSE cells express a unique combination of stress-resistance genes that allow them to survive in harsh environments where other cells would die. This includes resistance to inflammation, oxidative stress, and low oxygen conditions – all of which are common in damaged tissues.
Second, MUSE cells have enhanced migration abilities. They express higher levels of certain proteins that help cells move through tissues and respond to chemical attractants. This allows them to navigate through the body and locate sites of damage more effectively than other stem cells.
Third, MUSE cells have what scientists call "superior differentiation plasticity." This means they can more easily and completely transform into other cell types when given the appropriate signals. While other stem cells might only partially differentiate or retain some of their original characteristics, MUSE cells can fully commit to their new cellular identities.
Finally, MUSE cells seem to have enhanced paracrine effects – they secrete a variety of beneficial substances that help surrounding tissues heal even beyond the cells they directly replace. These substances can reduce inflammation, promote blood vessel formation, and stimulate the body's own repair mechanisms.
Challenges and Limitations
Despite their promise, MUSE cells face several challenges that researchers are working to address. One of the biggest challenges is standardization. Because MUSE cells are isolated from different donors and can vary in their properties, ensuring consistent quality and potency across different batches is crucial for reliable therapeutic outcomes.
Another challenge is determining optimal dosing and timing. How many MUSE cells should be given? How often should treatments be repeated? When is the best time to treat patients after injury or disease onset? These questions are being addressed through ongoing clinical trials, but definitive answers are still being developed.
Researchers are also working to better understand exactly how MUSE cells work at the molecular level. While their therapeutic effects are clear, the precise mechanisms by which they promote healing are still being unraveled. This understanding will be important for optimizing treatments and predicting which patients are most likely to benefit.
There's also the challenge of scaling up production to meet potential future demand. While MUSE cells are easier to produce than some other stem cell therapies, manufacturing them at the scale needed for widespread clinical use will require significant investment in specialized facilities and quality control systems.
Combination Therapies and Future Directions
One of the most exciting areas of MUSE cell research involves combining these cells with other treatments to enhance their effectiveness. Researchers are exploring combinations with growth factors, biomaterial scaffolds, and other regenerative medicine approaches.
For example, scientists are testing whether combining MUSE cells with specific growth factors can enhance their ability to differentiate into desired cell types. Others are investigating whether delivering MUSE cells along with biomaterial scaffolds can provide better support for tissue regeneration.
There's also interest in combining MUSE cell therapy with rehabilitation programs. The idea is that physical therapy, occupational therapy, or other rehabilitation approaches might work synergistically with MUSE cells to maximize functional recovery.
Gene editing technologies like CRISPR are also being explored as ways to potentially enhance MUSE cells. While MUSE cells are already highly effective in their natural state, researchers are investigating whether genetic modifications could make them even more powerful for treating specific conditions.
The Patient Experience
What is it actually like to receive MUSE cell therapy? Based on reports from clinical trial participants, the experience is relatively straightforward and similar to other intravenous treatments.
Patients typically receive the treatment as an outpatient procedure in a hospital or clinical setting. The MUSE cells are delivered through a standard IV line, usually over the course of 30 minutes to an hour. Most patients report little to no discomfort during the infusion itself.
In the hours and days following treatment, some patients experience mild flu-like symptoms, including fatigue, low-grade fever, or body aches. These symptoms are thought to reflect the immune system's recognition of the treatment and typically resolve within a few days.
The timeline for seeing benefits varies considerably. Some patients report improvements within weeks of treatment, while others may not see changes for several months. In some cases, improvements continue to occur for a year or more after treatment, suggesting that MUSE cells may continue working long after they're initially administered.
Regular follow-up appointments are typically required to monitor progress and watch for any side effects. These may include physical examinations, imaging studies, blood tests, and functional assessments depending on the condition being treated.
Cost and Accessibility
Currently, MUSE cell therapy is primarily available through clinical trials or in certain countries where it has received regulatory approval. For patients who can access the treatment commercially, costs typically range from tens of thousands to over $100,000, depending on the specific therapy and treatment protocol.
These high costs reflect the current limitations of small-scale production and the extensive quality control requirements for cellular therapies. However, as production scales up and manufacturing processes become more efficient, costs are expected to decrease significantly.
Several companies are working to make MUSE cell therapies more accessible by developing more efficient production methods and pursuing insurance coverage. Some are also exploring payment assistance programs or partnerships with healthcare systems to reduce costs for patients.
The global nature of MUSE cell development means that patients may have access to treatments in some countries before they become available in others. This has led to some medical tourism, though patients considering treatment abroad should carefully research the credentials of providers and the regulatory oversight of treatments.
Looking Ahead: The Future of MUSE Cell Therapy
The future of MUSE cell therapy looks incredibly promising. Current clinical trials are testing these cells for an expanding list of conditions, including diabetes, liver disease, kidney disease, and various forms of cancer. Early results suggest that MUSE cells may be effective for many of these conditions.
Researchers are also working on next-generation MUSE cell therapies that could be even more effective. These include cells that have been enhanced with specific proteins or growth factors, cells that have been genetically modified to produce therapeutic substances, and cells that are combined with advanced biomaterials.
The development of personalized MUSE cell therapies is another exciting possibility. While current therapies use cells from healthy donors, researchers are exploring whether MUSE cells could be isolated from patients' own tissues and used for autologous treatment. This approach could potentially offer even better outcomes with minimal risk of side effects.
Artificial intelligence and machine learning are also being applied to MUSE cell research to help identify which patients are most likely to benefit from treatment and to optimize treatment protocols. These technologies could help make MUSE cell therapy more precise and effective.
Perhaps most importantly, the success of MUSE cells is opening up new ways of thinking about regenerative medicine. Rather than trying to replace damaged tissues with artificial materials or mechanical devices, MUSE cells demonstrate the power of harnessing the body's own repair mechanisms.
As our understanding of MUSE cells continues to grow and as manufacturing processes become more efficient, these remarkable repair cells may become a standard treatment for a wide range of conditions that are currently difficult or impossible to treat. For patients facing devastating diagnoses, MUSE cells represent not just a new treatment option, but a new source of hope for recovery and healing.
The story of MUSE cells is still being written, but the early chapters suggest that these stress-enduring, self-guided repair specialists may indeed represent the future of regenerative medicine. As clinical trials continue and our understanding deepens, MUSE cells may transform from an experimental therapy into a mainstream medical treatment that helps millions of patients worldwide recover from injuries and diseases that were once considered incurable.