Maria Santos had been running marathons for fifteen years when her left knee began betraying her. What started as occasional stiffness after long runs gradually became constant pain that made even walking up stairs a challenge. The 42-year-old teacher and mother of two had built her identity around being active – weekend hikes with her family, coaching her daughter's soccer team, and the meditative rhythm of her morning runs that started each day.
When the MRI revealed significant cartilage damage in her knee, along with early signs of arthritis, Maria's orthopedic surgeon painted a stark picture. The cartilage that cushioned her knee joint had worn away in several areas, leaving bone rubbing against bone. Traditional treatments like physical therapy and anti-inflammatory medications might provide temporary relief, but the damage was irreversible. Her options were limited: learn to live with increasing pain and restrictions, or eventually undergo knee replacement surgery.
That consultation was eighteen months ago. Today, Maria is once again lacing up her running shoes for a 10-mile training run, preparing for her return to marathon competition. What changed? She became one of the first patients in her state to receive an innovative stem cell treatment that actually regenerated the cartilage in her damaged knee.
Maria's story represents a fundamental shift in how we approach joint problems and cartilage damage. For decades, orthopedic medicine has been largely about managing decline – helping patients cope with progressive joint deterioration while delaying the inevitable need for artificial joint replacement. Stem cell therapy is changing this paradigm by offering something that was previously impossible: the regeneration of healthy cartilage and the restoration of joint function.
The implications of this breakthrough extend far beyond individual success stories. Joint problems affect hundreds of millions of people worldwide, from weekend warriors dealing with sports injuries to elderly patients struggling with arthritis. Until now, medicine could offer pain relief, temporary fixes, and eventually joint replacement, but it couldn't address the underlying problem of damaged or missing cartilage. Stem cell therapy is beginning to change that fundamental limitation.
Understanding the Cartilage Challenge
To appreciate why stem cell therapy represents such a revolutionary advance for joint problems, it's crucial to understand what cartilage is, why it's so important, and why it has been so difficult to repair or replace.
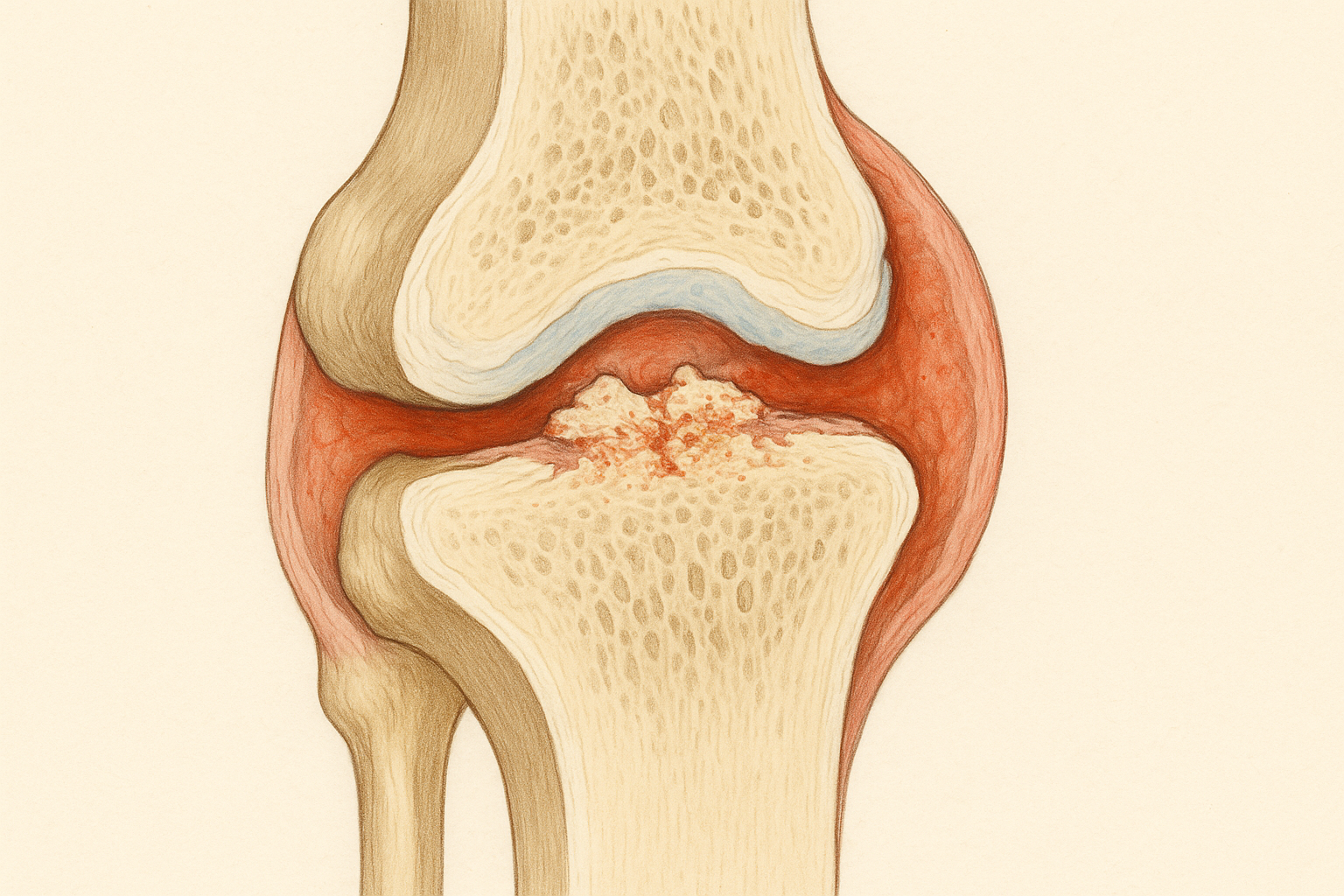
Cartilage is one of the most remarkable tissues in the human body, yet it's also one of the most vulnerable. This smooth, rubbery tissue covers the ends of bones in your joints, providing a nearly frictionless surface that allows bones to glide past each other during movement. Healthy cartilage is incredibly durable – it can withstand forces many times your body weight and perform flawlessly for decades.
The problem is that cartilage has virtually no blood supply. While this lack of blood vessels helps cartilage maintain its smooth structure and resist wear, it also means that damaged cartilage has very limited ability to heal itself. When you cut your skin, blood brings nutrients and healing cells to the wound. When cartilage is damaged, no such repair mechanism exists.
Cartilage damage can occur in several ways. Acute injuries from sports or accidents can tear or fragment cartilage. Repetitive use over time can cause gradual wear and breakdown. Genetic factors can predispose some people to cartilage problems, while excess weight puts additional stress on joint surfaces. Inflammatory conditions like arthritis can accelerate cartilage destruction.
Once cartilage is significantly damaged, a cascade of problems typically follows. The smooth joint surface becomes rough and irregular, causing pain and stiffness. The underlying bone may be exposed, leading to additional inflammation and pain. The joint may lose its normal alignment and stability. Over time, these changes usually worsen, leading to progressive disability and the eventual need for joint replacement surgery.
Traditional treatments for cartilage damage have focused on managing symptoms rather than addressing the underlying problem. Anti-inflammatory medications can reduce pain and swelling but don't repair damaged tissue. Physical therapy can help maintain mobility and strengthen supporting muscles but can't restore missing cartilage. Injections of lubricants or steroids can provide temporary relief but don't promote healing.
Surgical options have been similarly limited. Procedures like arthroscopy can remove damaged cartilage fragments and smooth rough surfaces, but they actually reduce the total amount of cartilage in the joint. Techniques like microfracture can stimulate some healing response, but they typically produce fibrocartilage, which is inferior to the original hyaline cartilage and tends to wear out quickly.
Joint replacement surgery, while highly successful for end-stage arthritis, is essentially an admission that the joint cannot be saved. While artificial joints can restore function and eliminate pain, they have limited lifespans, may restrict certain activities, and carry surgical risks that make them inappropriate for younger or more active patients.
The Science of Cartilage Regeneration
Stem cell therapy approaches cartilage repair from an entirely different perspective. Instead of accepting that damaged cartilage cannot heal, researchers have been working to provide joints with the cellular tools they need to regenerate healthy tissue.
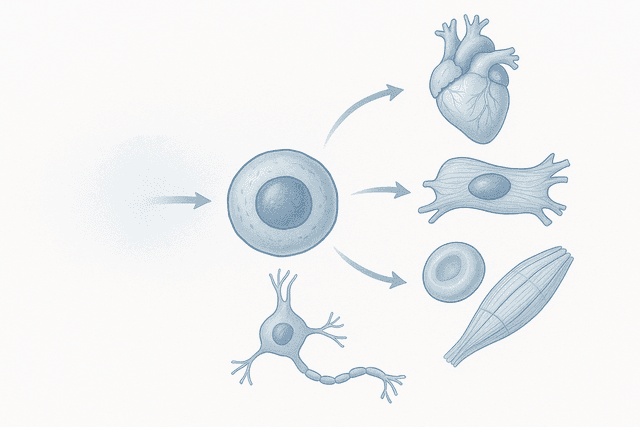
The process of cartilage regeneration using stem cells involves several complex steps, each of which has required years of research to understand and optimize. The first challenge is obtaining the right type of stem cells. Different types of stem cells have different capabilities for becoming cartilage, and researchers have tested cells from various sources including bone marrow, fat tissue, synovial fluid, and even embryonic sources.
Mesenchymal stem cells (MSCs) have emerged as the most promising type for cartilage repair. These cells can be obtained from several sources in the patient's own body, eliminating concerns about immune rejection. MSCs have the natural ability to differentiate into cartilage cells called chondrocytes, but they can also contribute to healing through other mechanisms.
The second challenge is delivering stem cells to the damaged area in a way that promotes cartilage formation rather than scar tissue or other unwanted tissue types. Simply injecting stem cells into a joint doesn't guarantee that they will form cartilage. The cells need the right environment, including appropriate growth factors, mechanical signals, and structural support.
Researchers have developed various approaches to optimize the environment for cartilage regeneration. Some treatments combine stem cells with growth factors that specifically promote cartilage formation. Others use scaffolds or matrices that provide structural support and guide tissue development. Still others modify the stem cells before injection to enhance their cartilage-forming capabilities.
The third challenge is ensuring that newly formed cartilage integrates properly with existing tissue and functions like normal cartilage. This requires not just the formation of cartilage-like tissue, but the development of the complex three-dimensional structure and biochemical composition that gives cartilage its unique properties.
Recent advances in understanding cartilage biology have provided crucial insights into how to optimize stem cell therapy for joint repair. Scientists have identified the specific signals that guide stem cells to become cartilage, the factors that promote cartilage maturation, and the conditions that help new cartilage integrate with existing tissue.
Perhaps most importantly, researchers have learned that successful cartilage regeneration often requires addressing not just the damaged cartilage itself, but the entire joint environment. This includes reducing inflammation, optimizing mechanical loading, and sometimes treating problems in the underlying bone or surrounding soft tissues.
Different Types of Orthopedic Stem Cell Treatments
The field of orthopedic stem cell therapy encompasses several distinct approaches, each targeting different types of joint problems and using different methods to promote healing. Understanding these various treatment options helps explain why results can vary and why some approaches may be more suitable for certain conditions than others.
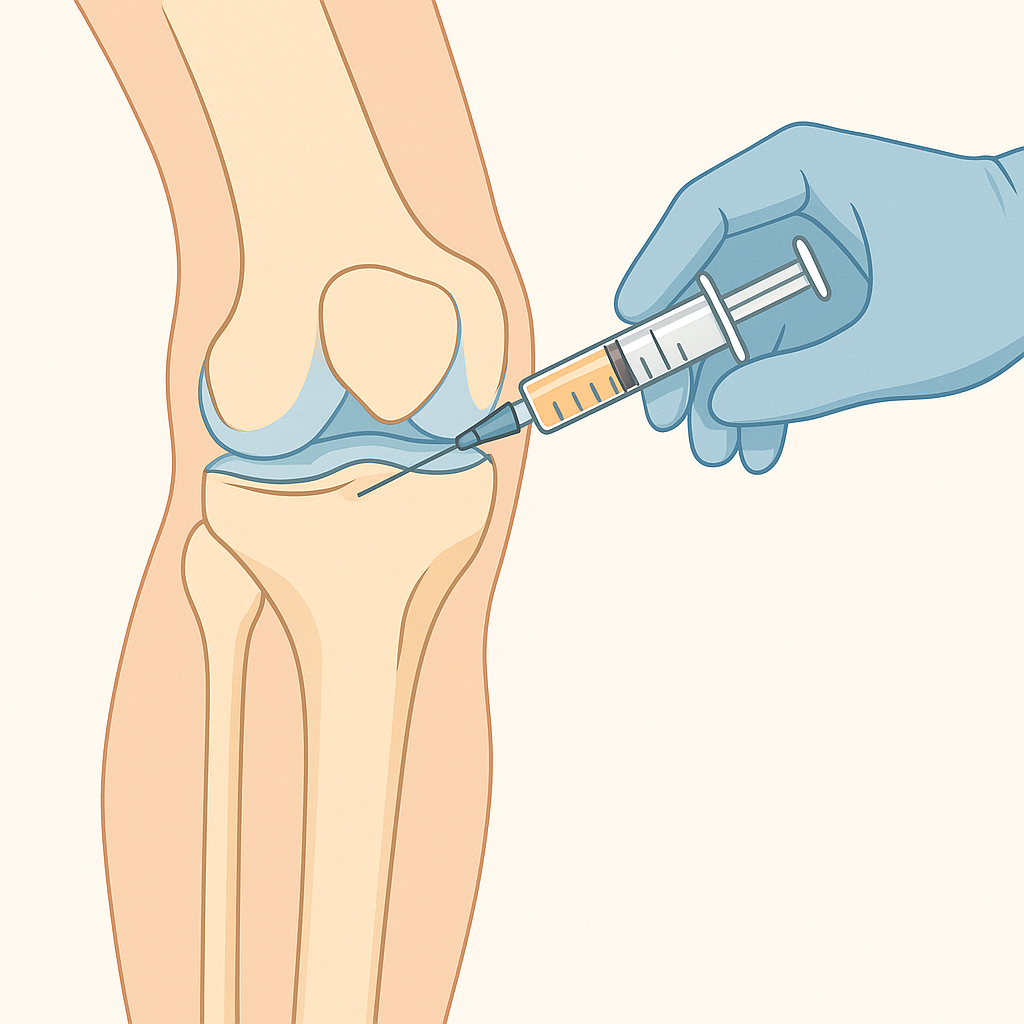
Autologous stem cell therapy uses the patient's own stem cells, typically harvested from bone marrow or fat tissue. This approach has the advantage of avoiding immune rejection and can usually be performed as a same-day procedure. The stem cells are extracted, processed to concentrate them, and then injected into the damaged joint, often with imaging guidance to ensure precise placement.
The bone marrow aspiration procedure typically involves inserting a needle into the hip bone and withdrawing a small amount of bone marrow, which contains MSCs along with other beneficial cells and growth factors. The entire sample may be used, or the stem cells may be concentrated using centrifugation or other processing techniques.
Fat-derived stem cell therapy involves removing a small amount of fat tissue, usually through a minor liposuction procedure, and then processing it to isolate MSCs. Some studies suggest that fat tissue may contain higher concentrations of MSCs than bone marrow, and the extraction procedure is generally less uncomfortable for patients.
Allogeneic stem cell therapy uses stem cells from donors rather than the patient's own cells. These treatments typically use cells from young, healthy donors that have been extensively tested for safety and potency. While there are theoretical concerns about immune rejection, many joints appear to be relatively immune-privileged sites where foreign cells can function without causing significant immune reactions.
Expanded stem cell therapy involves taking a small sample of the patient's stem cells and growing them in laboratory conditions to create much larger numbers of cells before treatment. This approach can provide many more stem cells than can be obtained directly from the patient, potentially enhancing treatment effectiveness. However, the process takes several weeks and adds complexity and cost to the treatment.
Combination therapies use stem cells along with other substances to enhance their effectiveness. Some treatments combine stem cells with platelet-rich plasma (PRP), which contains growth factors that may promote healing. Others add hyaluronic acid, which can provide lubrication and may create a better environment for stem cell function. Still others incorporate scaffolds or matrices that provide structural support for tissue regeneration.
Advanced cell therapies use stem cells that have been modified or enhanced before treatment. Some approaches pre-treat stem cells with growth factors to "prime" them for cartilage formation. Others use genetic modification to enhance the cells' regenerative capabilities. These more sophisticated approaches are typically available only in research settings but may represent the future of orthopedic stem cell therapy.
Each approach has advantages and limitations, and the optimal choice depends on factors including the type and extent of joint damage, the patient's age and health status, and the specific capabilities and experience of the treatment center.
Clinical Evidence and Success Rates
The evidence supporting orthopedic stem cell therapy has grown substantially over the past decade, with numerous clinical trials documenting both the potential benefits and limitations of various approaches. Understanding this evidence is crucial for patients considering treatment and for setting realistic expectations about outcomes.
Early clinical trials using basic stem cell injections showed modest but meaningful improvements in joint pain and function. A typical study might show that 60-70% of patients experienced some degree of improvement, with average pain reductions of 30-50% and functional improvements that allowed patients to return to activities they had given up due to joint problems.
More recent studies using refined techniques and better patient selection have reported higher success rates. Some trials using concentrated bone marrow stem cells for knee arthritis have reported success rates of 80% or higher, with improvements maintained for two years or more. Studies using fat-derived stem cells have shown similar results.
The most impressive results have come from studies using combination approaches or more advanced cell preparation techniques. Trials combining stem cells with growth factors or scaffolds have reported success rates approaching 90% for certain types of cartilage defects, with some patients showing actual regeneration of cartilage tissue on MRI scans.
However, it's important to note that results vary significantly depending on several factors. Younger patients generally respond better than older patients. Smaller areas of damage typically heal better than extensive cartilage loss. Patients with isolated cartilage defects often do better than those with widespread arthritis. The skill and experience of the treatment team also appears to influence outcomes significantly.
Long-term follow-up studies have provided important insights into the durability of stem cell therapy benefits. While some patients maintain improvements for years, others experience gradual decline in benefits over time. Factors that appear to predict better long-term outcomes include younger age, lower body weight, higher activity levels, and absence of significant joint malalignment.
Imaging studies have provided objective evidence of cartilage regeneration in some patients treated with stem cell therapy. MRI scans can detect new cartilage formation, and some studies have used specialized MRI techniques to assess the quality of regenerated tissue. While not all patients who experience clinical improvement show clear evidence of cartilage regeneration on imaging, those who do tend to have more durable improvements.
Comparative studies have begun to provide insights into which approaches work best for different types of problems. For example, some studies suggest that fat-derived stem cells may be more effective for certain types of arthritis, while bone marrow stem cells may work better for acute sports injuries. Combination therapies generally appear to produce better results than stem cells alone.
Sports Medicine Applications
Stem cell therapy has found particularly compelling applications in sports medicine, where athletes and active individuals seek treatments that can restore function rather than just manage symptoms. The unique demands of sports medicine – where patients often want to return to high-level activities rather than just achieve basic function – have driven many innovations in orthopedic stem cell therapy.
Professional and collegiate athletes have been among the early adopters of stem cell therapy, often willing to try experimental treatments that might allow them to continue their careers. While specific patient information is often confidential, numerous reports describe athletes returning to competition after stem cell treatments for injuries that might otherwise have been career-ending.
The types of sports injuries most commonly treated with stem cell therapy include cartilage defects in the knee, chronic tendon problems like tennis elbow or Achilles tendinitis, muscle strains that haven't healed properly, and early arthritis in young athletes. Each of these conditions presents unique challenges and may require different treatment approaches.
Cartilage injuries in athletes often occur during pivoting sports like soccer, basketball, or skiing. These injuries can be particularly devastating for young athletes because traditional treatments often don't restore the high level of function needed for sports participation. Stem cell therapy offers the possibility of actually regenerating cartilage and returning athletes to their pre-injury level of performance.
Tendon injuries represent another area where stem cell therapy has shown promise. Chronic tendon problems like tennis elbow or jumper's knee can be particularly frustrating because they often don't respond well to conventional treatments and can persist for months or years. Stem cell therapy may help promote healing in these chronic conditions by providing growth factors and cells that can repair damaged tendon tissue.
The treatment protocols for athletes often differ from those used for general patients. Athletes may receive more aggressive treatments, including higher concentrations of stem cells or combination therapies with multiple growth factors. The rehabilitation protocols are also typically more intensive, taking advantage of athletes' high motivation and access to specialized training facilities.
However, sports medicine applications of stem cell therapy also face unique challenges. Athletes often have higher expectations for treatment outcomes and may be more willing to take risks for the chance of complete recovery. The pressure to return to competition quickly can sometimes lead to premature return to activity before healing is complete.
Professional sports organizations have had to develop policies regarding stem cell therapy use, balancing athlete welfare with competitive fairness concerns. Most major sports leagues now allow stem cell therapy using the athlete's own cells, but some restrict the use of certain growth factors or enhancement techniques.
The success stories from sports medicine have helped drive broader acceptance of orthopedic stem cell therapy. When high-profile athletes return to competition after stem cell treatment, it demonstrates the potential of these therapies and encourages further research and development.
Arthritis Treatment: Managing Expectations
Arthritis represents perhaps the most challenging application for orthopedic stem cell therapy, but also potentially the most impactful given the millions of people affected by various forms of joint arthritis. The relationship between stem cell therapy and arthritis is complex, and understanding this relationship is crucial for setting appropriate expectations.
Osteoarthritis, the most common form of arthritis, involves the gradual breakdown of cartilage along with changes in the underlying bone and surrounding soft tissues. This process typically occurs over years or decades and affects multiple aspects of joint function. Stem cell therapy for osteoarthritis aims to slow or reverse this degenerative process, but the extent to which this is possible depends on several factors.
Early-stage osteoarthritis, where cartilage damage is limited and joint structure is still relatively normal, appears to respond best to stem cell therapy. In these cases, the treatment may help repair minor cartilage defects, reduce inflammation, and potentially prevent or slow further degeneration. Some patients with early arthritis have experienced significant symptom relief and functional improvement that has lasted for years.
Moderate osteoarthritis, where there is more extensive cartilage loss but the joint structure is still intact, presents a more challenging scenario. Stem cell therapy may still provide benefits, but complete restoration of joint function is less likely. The treatment may help with pain relief, improve function modestly, and potentially slow the progression of arthritis, but patients typically need to maintain realistic expectations about the degree of improvement possible.
Advanced osteoarthritis, where there is severe cartilage loss, bone-on-bone contact, and significant joint deformity, is the most challenging condition to treat with stem cell therapy. While some patients with advanced arthritis have experienced meaningful improvements, the treatment is less likely to provide dramatic benefits. In these cases, stem cell therapy might be considered as a way to delay joint replacement surgery or improve function modestly, but it's not typically a substitute for joint replacement.
Rheumatoid arthritis and other inflammatory forms of arthritis present different challenges for stem cell therapy. These conditions involve active inflammation that can destroy joints rapidly, and stem cell therapy must address both the inflammatory process and the resulting joint damage. Some studies have suggested that certain types of stem cells may have anti-inflammatory properties that could benefit patients with inflammatory arthritis, but the evidence is still preliminary.
The timing of stem cell therapy for arthritis appears to be crucial. Treatment earlier in the disease process, before extensive joint damage has occurred, is more likely to be successful. This has led some experts to advocate for stem cell therapy as a preventive treatment for people at high risk of developing arthritis, though this approach requires more research.
Patient selection is particularly important for arthritis applications of stem cell therapy. Factors like age, weight, activity level, and the presence of joint malalignment all influence treatment outcomes. Younger, more active patients with normal joint alignment typically respond better than older, sedentary patients with significant joint deformity.
The combination of stem cell therapy with other arthritis treatments is an active area of research. Some studies are exploring whether combining stem cell therapy with anti-inflammatory medications, physical therapy, or other interventions can improve outcomes. The goal is to create comprehensive treatment programs that address all aspects of the arthritic process.
The Treatment Experience
For patients considering orthopedic stem cell therapy, understanding what the treatment experience involves can help with decision-making and preparation. While specific procedures vary depending on the type of stem cell therapy and the joint being treated, there are common elements to most orthopedic stem cell treatments.
The process typically begins with comprehensive evaluation to determine if a patient is a good candidate for stem cell therapy. This includes detailed medical history, physical examination, imaging studies like X-rays or MRI scans, and sometimes specialized tests to assess joint function. The evaluation helps determine the type and extent of joint damage and whether stem cell therapy is likely to be beneficial.
For treatments using the patient's own stem cells, the next step is harvesting the stem cell source. Bone marrow aspiration is typically performed in an outpatient setting using local anesthesia. A needle is inserted into the hip bone, and a small amount of bone marrow is withdrawn. The procedure usually takes about 15-30 minutes and causes discomfort similar to a blood draw, though some patients experience aching in the hip for a day or two afterward.
Fat harvesting for adipose-derived stem cell therapy involves a minor liposuction procedure, usually performed on the abdomen or thigh. Local anesthesia is used, and a small amount of fat tissue is removed through a thin tube. The procedure typically takes 30-45 minutes and causes minimal discomfort, though some patients experience bruising or swelling at the harvest site.
The harvested tissue is then processed to isolate and concentrate the stem cells. This processing can take anywhere from 30 minutes to several hours, depending on the specific technique used. Some treatments use the entire bone marrow or fat sample, while others separate out specific cell populations or concentrate the stem cells to higher levels.
The injection procedure itself is typically performed using imaging guidance to ensure accurate placement of the stem cells. Ultrasound or fluoroscopy (live X-ray) guidance helps the physician direct the injection precisely to the area of damage. The injection usually takes just a few minutes and causes discomfort similar to other joint injections.
Recovery from the injection varies depending on the joint treated and the specific procedure used. Most patients can return to normal daily activities within a day or two, though they may be advised to avoid strenuous activities for a period of time. Some patients experience increased pain or stiffness for a few days after treatment as part of the healing response.
The timeline for seeing benefits from stem cell therapy is typically longer than with conventional treatments. While some patients notice improvements within weeks, others may not see significant changes for several months. The healing process can continue for six months to a year or more, with some patients experiencing progressive improvement over this extended period.
Follow-up care typically includes regular check-ups to monitor progress and may include physical therapy to optimize the healing response. Some treatment protocols include multiple injections spaced several months apart, while others involve single treatments with long-term follow-up.
Global Perspectives and Regulatory Landscape
The regulatory environment for orthopedic stem cell therapy varies significantly around the world, creating different opportunities and challenges for patients seeking treatment. Understanding this global landscape can help patients navigate their options and make informed decisions about where and how to access treatment.
In the United States, the FDA regulates stem cell therapies as biological products, requiring extensive clinical trials and approval processes for most treatments. This rigorous oversight helps ensure safety and efficacy but also means that many promising treatments remain in clinical trials and aren't widely available. However, certain types of stem cell procedures using minimally manipulated autologous cells may be performed under the FDA's same-day treatment guidelines.
The regulatory environment has led to a complex situation where some stem cell treatments are available at specialized medical centers, while others remain experimental. Patients may be able to access certain treatments through clinical trials or at centers that operate under specific regulatory exemptions, but the availability varies significantly by location and specific medical condition.
Europe has a somewhat different regulatory approach, with the European Medicines Agency providing oversight while allowing individual countries some flexibility in their specific regulations. Some European countries have been more permissive of certain stem cell treatments, while others have taken more restrictive approaches similar to the United States.
Japan has emerged as a leader in regenerative medicine regulation, with legislation that allows conditional approval of certain stem cell treatments based on preliminary evidence while requiring longer-term studies to confirm benefits. This approach has made some advanced stem cell therapies available to Japanese patients before they receive approval in other countries.
Several countries in Asia and Latin America have developed significant stem cell therapy industries with varying levels of regulatory oversight. Some offer treatments that aren't available elsewhere, though patients considering treatment abroad should carefully research the credentials of providers and the regulatory oversight of treatments.
The global nature of stem cell research has led to international collaborations and sharing of research findings, which has accelerated progress in the field. However, it has also created challenges for patients trying to understand which treatments are proven effective and which remain experimental.
Medical tourism for stem cell therapy has become increasingly common, with patients traveling to other countries to access treatments that aren't available at home. While this can provide access to innovative treatments, it also carries risks including variable quality of care, limited follow-up support, and potential complications from traveling after treatment.
Professional medical organizations have developed guidelines to help patients and physicians navigate the complex landscape of stem cell therapy options. These guidelines emphasize the importance of evidence-based treatment, appropriate patient selection, and comprehensive informed consent processes.
Economic Considerations and Insurance Coverage
The cost of orthopedic stem cell therapy varies widely depending on the specific treatment approach, the medical center providing care, and geographic location. Understanding these financial considerations is important for patients contemplating treatment, as most stem cell therapies are not currently covered by insurance.
Simple stem cell injections using basic processing techniques may cost anywhere from $3,000 to $8,000 per treatment. More sophisticated approaches involving cell expansion, combination therapies, or multiple injections can cost $15,000 to $25,000 or more. These costs typically include the cell harvesting procedure, processing, injection, and initial follow-up care.
The cost-effectiveness of stem cell therapy depends largely on its success in avoiding or delaying more expensive treatments like joint replacement surgery. A knee replacement surgery can cost $35,000 to $50,000 or more, not including the costs of rehabilitation and potential complications. If stem cell therapy can delay joint replacement by several years or eliminate the need for it entirely, it may ultimately be cost-effective despite the high upfront costs.
Insurance coverage for orthopedic stem cell therapy is currently very limited. Most insurance companies consider the treatments experimental and don't provide coverage. However, this situation is beginning to change as some treatments demonstrate clear benefits in clinical trials and receive regulatory approval.
Some medical centers offer financing options or payment plans to help make treatments more accessible. Patient advocacy organizations sometimes provide grants or other financial assistance for specific conditions. It's worth exploring all options with treatment providers and insurance companies, as policies are evolving.
The development of standardized treatment protocols and more efficient cell processing techniques may help reduce costs over time. Competition between different treatment approaches and providers may also drive down prices as the field matures and treatments become more widely available.
Some employers and specialized insurance products are beginning to cover certain types of stem cell therapy, particularly for younger employees or athletes where the treatments may prevent more expensive long-term problems. Workers' compensation insurance may cover stem cell therapy for work-related injuries in some jurisdictions.
The economics of stem cell therapy also need to be considered in the context of quality of life improvements. For patients who regain the ability to work, exercise, or participate in activities they had given up due to joint problems, the value of treatment may extend far beyond the direct medical costs and savings.
Combining Stem Cell Therapy with Conventional Treatments
One of the most promising developments in orthopedic stem cell therapy is the integration of these treatments with conventional approaches to create comprehensive treatment programs. Rather than viewing stem cell therapy as an alternative to traditional treatments, many experts now see it as a complementary approach that can enhance the effectiveness of established therapies.
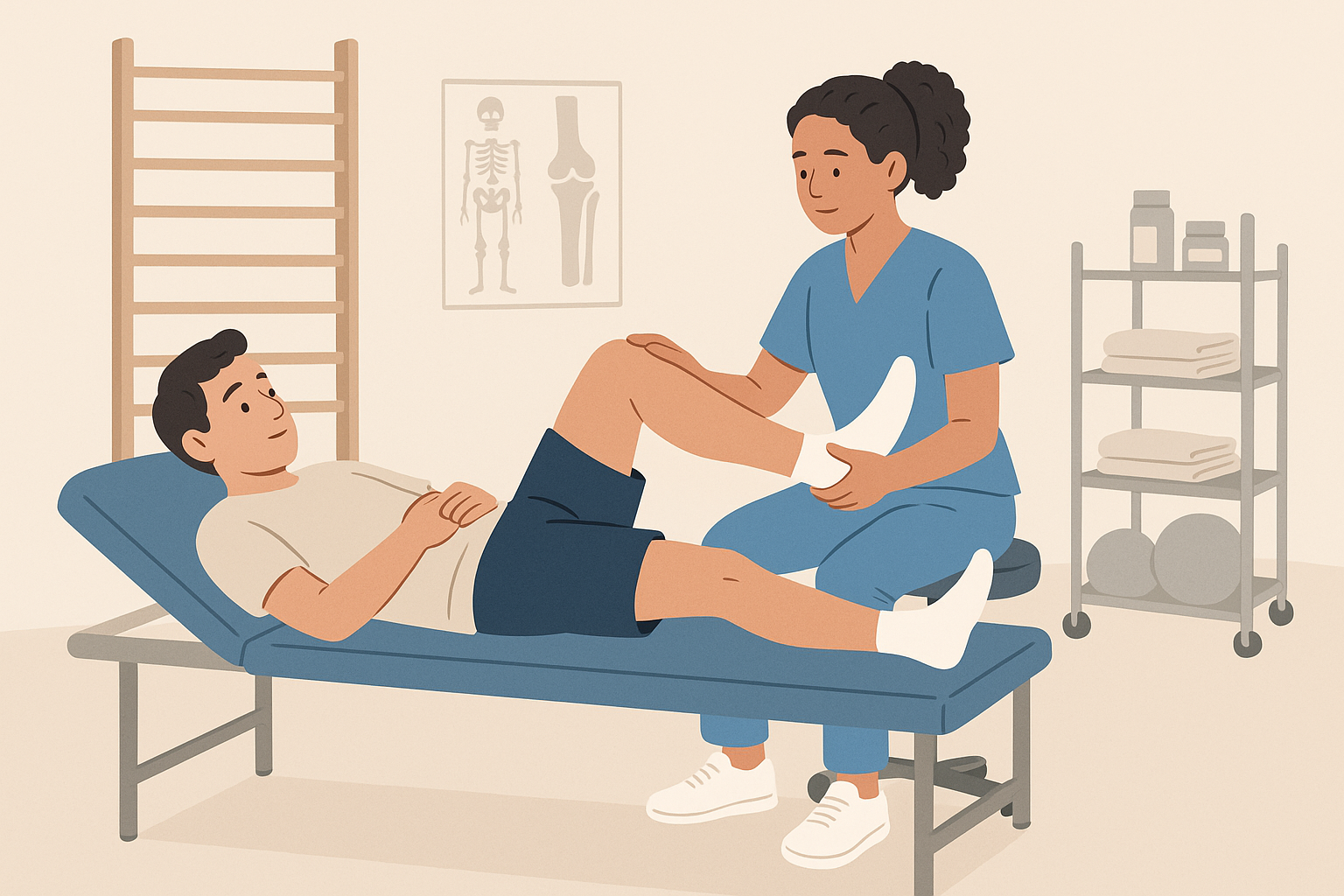
Physical therapy plays a crucial role in optimizing outcomes from stem cell therapy. The mechanical stimulation provided by appropriate exercise can help guide stem cells toward forming the right type of tissue and can promote the integration of new tissue with existing structures. However, the timing and intensity of physical therapy after stem cell treatment requires careful consideration to avoid disrupting the healing process.
Some treatment protocols recommend a period of limited activity immediately after stem cell injection to allow the cells to establish themselves in the joint. This is typically followed by a gradual increase in activity under the guidance of physical therapists who understand the unique requirements of stem cell healing.
Nutritional support may also play a role in optimizing stem cell therapy outcomes. Certain nutrients are important for cartilage synthesis and stem cell function, and some treatment centers provide specific nutritional guidance or supplements to support the healing process. While the evidence for specific nutritional interventions is still limited, maintaining good overall nutrition is clearly important for optimal healing.
Anti-inflammatory medications present a complex consideration in stem cell therapy. While reducing inflammation may create a better environment for stem cell function, some anti-inflammatory drugs might interfere with the natural healing processes that stem cell therapy aims to promote. Treatment protocols typically provide specific guidance about which medications to continue, modify, or avoid during the healing period.
Weight management is another important component of comprehensive joint care that may influence stem cell therapy outcomes. Excess weight places additional stress on joints and may interfere with healing processes. Some treatment centers require patients to achieve certain weight targets before undergoing stem cell therapy, while others incorporate weight management programs into their treatment protocols.
Biomechanical factors like joint alignment and movement patterns can significantly influence the success of stem cell therapy. Patients with significant joint malalignment may need corrective procedures either before or in conjunction with stem cell treatment. Similarly, addressing abnormal movement patterns through physical therapy or other interventions may be necessary to prevent re-injury of regenerated tissue.
The integration of stem cell therapy with other regenerative medicine approaches is an active area of research. Some studies are exploring combinations with platelet-rich plasma, growth factor injections, or other biological treatments. The goal is to create synergistic effects that produce better outcomes than any single treatment alone.
Future Directions and Emerging Technologies
The field of orthopedic stem cell therapy continues to evolve rapidly, with new approaches and technologies being developed that may overcome current limitations and expand treatment options for joint problems.
Tissue engineering approaches are becoming increasingly sophisticated, with researchers working to create three-dimensional cartilage constructs that can be implanted to repair large defects. These engineered tissues are grown in laboratory conditions using stem cells and specialized scaffolds that provide the structure and signals needed for proper cartilage development.
Gene therapy approaches are being explored as ways to enhance stem cell function or to directly promote cartilage regeneration. Some experimental treatments involve modifying stem cells to produce specific growth factors or other therapeutic proteins. Others use gene therapy to enhance the joint's own regenerative capabilities.
Nanotechnology is being applied to improve stem cell delivery and function. Nanoparticles can be used to deliver drugs or growth factors specifically to stem cells, while nanosensors might eventually allow real-time monitoring of stem cell function and tissue regeneration.
3D bioprinting technology is being developed to create complex joint tissues with precise cellular organization. This approach could potentially create replacement cartilage or even entire joint surfaces that match the patient's anatomy exactly.
Artificial intelligence and machine learning are being used to optimize stem cell therapy protocols. These technologies can analyze complex patterns in patient data, treatment outcomes, and tissue development to identify the most effective approaches for different types of patients and joint problems.
Combination therapies using multiple types of stem cells or stem cells combined with other regenerative approaches are showing promise in early studies. The goal is to recreate the complex cellular environment needed for optimal joint repair and regeneration.
Personalized medicine approaches are becoming more feasible as our understanding of individual factors that influence treatment outcomes improves. This could lead to treatments that are specifically tailored to each patient's unique genetic makeup, injury pattern, and healing capacity.
Making Treatment Decisions
Deciding whether to pursue orthopedic stem cell therapy requires careful consideration of multiple factors and should involve consultation with qualified medical professionals who understand both the patient's specific condition and the current state of stem cell therapy.
The first consideration is whether stem cell therapy is appropriate for the specific type and severity of joint problem. This assessment requires detailed evaluation of the joint damage, the patient's symptoms and functional limitations, and the likelihood that stem cell therapy will provide meaningful benefits.
Understanding realistic expectations is crucial. While some patients experience dramatic improvements from stem cell therapy, others see modest benefits or no improvement at all. The likelihood of significant benefit depends on factors including the type and extent of joint damage, the patient's age and overall health, and the specific treatment approach used.
The availability and appropriateness of conventional treatment options should also be considered. For patients who haven't tried standard treatments like physical therapy, medications, or conventional injections, these approaches might be more appropriate as initial therapy. For others who have exhausted conventional options, stem cell therapy may represent one of the few remaining alternatives to major surgery.
Risk tolerance is an important personal factor. Stem cell therapy is generally safe, but it does carry some risks and uncertainties. Some patients are comfortable with these uncertainties for the chance of improvement, while others prefer to wait for more established treatments.
Access to high-quality treatment is essential. Stem cell therapy should only be performed by experienced medical professionals at reputable medical centers with appropriate oversight and quality controls. The field unfortunately includes some providers who make exaggerated claims or use unproven techniques, so careful research of providers is important.
Financial considerations are significant for most patients, given the limited insurance coverage for stem cell therapy. Understanding the full costs of treatment, including follow-up care, and exploring all available financing options is important for making informed decisions.
The decision should be made in consultation with orthopedic specialists who understand both the patient's specific condition and the current evidence for stem cell therapy. Seeking multiple opinions can be valuable, particularly for complex cases or when considering experimental treatments.
The Changing Landscape of Joint Care
Orthopedic stem cell therapy is fundamentally changing how we think about joint problems and the possibilities for maintaining joint health throughout life. This transformation extends beyond the treatments themselves to influence all aspects of joint care.
The traditional approach to joint problems has been largely reactive – waiting for significant damage to occur and then managing the consequences. Stem cell therapy is enabling a more proactive approach, where early intervention might prevent or reverse joint damage before it becomes severe.
This shift is influencing how joint problems are diagnosed and monitored. Advanced imaging techniques that can detect early cartilage changes are becoming more important, as are biomarkers that might identify joint problems before symptoms appear. The goal is to identify and treat joint problems at a stage where regenerative therapies are most likely to be successful.
The success of stem cell therapy is also driving innovation in other areas of joint care. If joints can be prompted to heal with the right cellular support, what other interventions might promote joint health? This question is spurring research into new drugs, nutritional approaches, exercise programs, and lifestyle interventions.
Perhaps most importantly, stem cell therapy is providing hope to patients with joint problems. Even for those who don't yet have access to these treatments, knowing that research is actively working toward regenerating damaged joints provides psychological benefits and motivation to maintain joint health while waiting for treatments to become available.
The field still faces significant challenges. Not all patients respond to treatment, optimal protocols are still being developed, and long-term outcomes are still being studied. The costs of treatment remain high, and insurance coverage is limited. Regulatory pathways need to balance innovation with safety requirements.
However, the progress made in just the past decade has been remarkable. Treatments that seemed impossible just a few years ago are now helping real patients avoid joint replacement surgery and return to activities they thought they had lost forever. As research continues and technologies improve, the potential for stem cell therapy to transform joint care becomes increasingly clear.
For patients like Maria Santos, who are dealing with joint problems today, stem cell therapy represents more than just a medical treatment – it represents the possibility that joint damage doesn't have to be permanent and that active, pain-free living may be possible even after significant joint injury.
The future of orthopedic stem cell therapy looks bright, with new approaches entering clinical trials regularly and existing treatments being refined and improved. As our understanding of joint biology and regeneration grows and our ability to harness stem cells becomes more sophisticated, we may be entering an era where joint problems become increasingly treatable and perhaps even preventable.
This transformation won't happen overnight, and there will undoubtedly be setbacks and challenges along the way. But for the first time in medical history, we have scientific tools that can actually regenerate damaged joint tissue and restore lost function. That represents a fundamental shift in the fight against joint disease and offers genuine hope to millions of patients and families worldwide.