Transforming Medicine
Heart failure remains one of medicine's most formidable challenges, affecting over 64 million people worldwide and carrying a 5-year mortality rate that exceeds many cancers. Despite advances in pharmacological therapies and devices, conventional treatments primarily manage symptoms rather than addressing the fundamental problem: the heart's limited capacity to regenerate damaged tissue.
In early 2025, this longstanding paradigm began to shift dramatically with groundbreaking clinical trial results published in Nature demonstrating that engineered stem cell patches could strengthen failing hearts and restore function in select patients. This landmark study represented the culmination of years of translational research and opened a new chapter in cardiovascular medicine.
Unlike traditional stem cell therapies that involve direct injection of cells into damaged tissue—where most cells die or wash away before integrating—cardiac patches represent an innovative approach that combines stem cells with supportive biomaterials to create living tissue constructs. These patches provide a nurturing environment for cells and serve as a structural scaffold to facilitate repair of damaged heart muscle.
This article explores the remarkable journey of stem cell patches from laboratory concept to clinical reality, examining their composition, mechanisms of action, recent breakthroughs, and future potential to transform heart failure treatment.
Understanding Cardiac Patches: Composition and Design
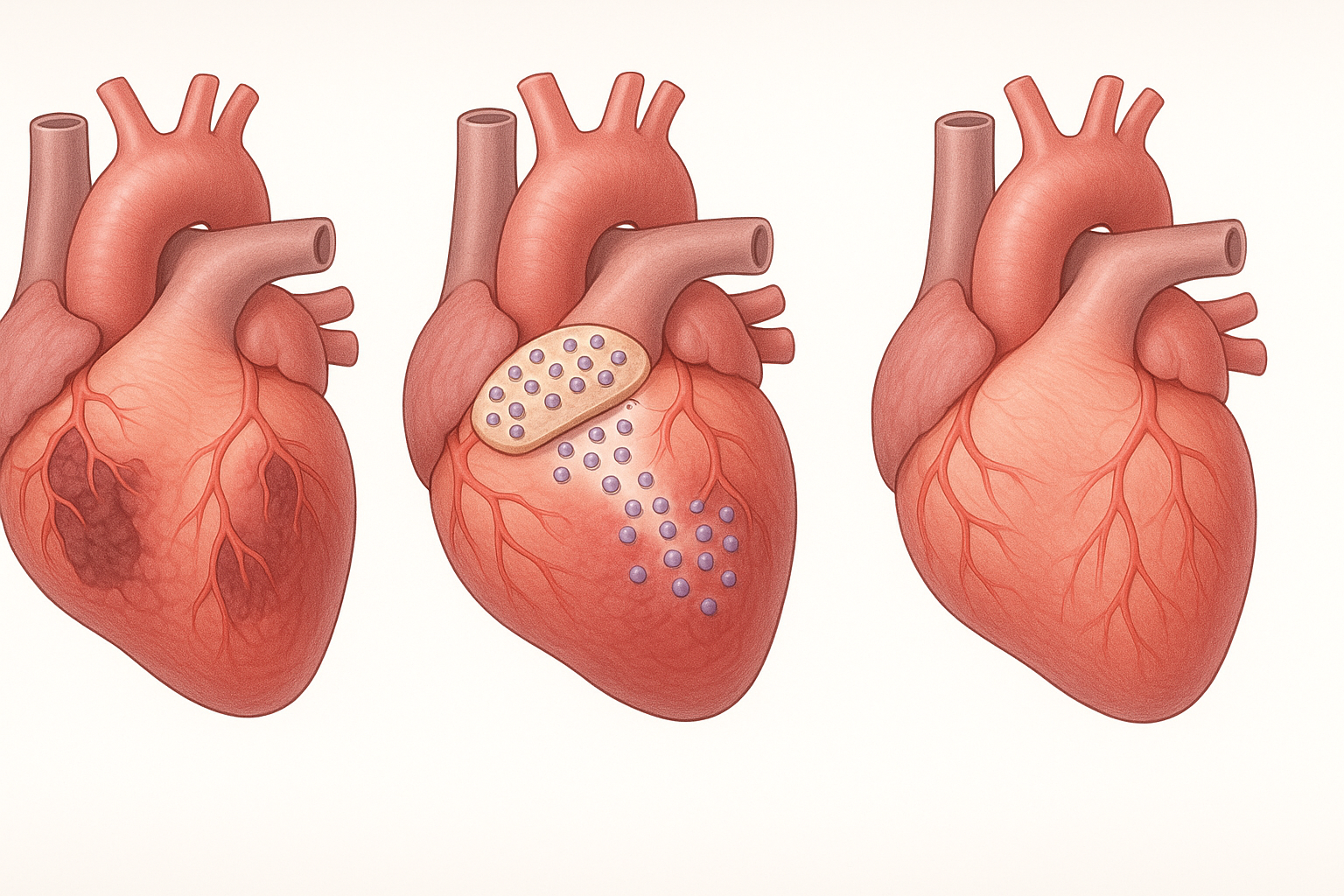
At their core, cardiac stem cell patches are sophisticated tissue engineering constructs designed to promote heart repair. Their development represents a convergence of stem cell biology, materials science, and surgical innovation. Modern patches typically incorporate several key components:
Cellular Components
The cellular component of cardiac patches has evolved significantly over time, with researchers exploring various cell types to identify those with optimal regenerative properties:
-
Mesenchymal Stem Cells (MSCs): Early patches primarily utilized MSCs derived from bone marrow or adipose tissue. While these cells produce beneficial paracrine factors, their limited cardiomyogenic potential led researchers to explore alternative cell sources.
-
Cardiac Progenitor Cells (CPCs): More recent approaches have incorporated cardiac-specific progenitor cells that demonstrate greater capacity to differentiate into functioning cardiomyocytes. A study published in Cell Stem Cell in February 2025 found that patches containing CPCs showed 3.5 times higher engraftment rates than those with MSCs.
-
Induced Pluripotent Stem Cell-Derived Cardiomyocytes (iPSC-CMs): The latest generation of patches utilizes cardiomyocytes derived from induced pluripotent stem cells. These cells more closely resemble native heart cells, demonstrating appropriate electrical activity and contractile properties. Research published in Science Translational Medicine in March 2025 demonstrated that iPSC-CM patches established electrical coupling with host tissue in porcine models, a critical factor for functional integration.
-
Multi-Cell Approaches: Cutting-edge patches now often incorporate multiple cell types, including endothelial cells and smooth muscle cells alongside cardiomyocytes, to recapitulate the complex cellular environment of heart tissue and promote vascularization.
A comparative analysis published in Circulation Research in January 2025 evaluated outcomes across different cell compositions, finding that tri-lineage patches (containing cardiomyocytes, endothelial cells, and smooth muscle cells) outperformed single-cell-type patches in terms of vascularization, tissue integration, and functional improvement.
Biomaterial Scaffolds
The choice of biomaterial scaffold is crucial for patch performance, providing structural support while facilitating cell survival, organization, and integration. Several approaches have shown promise:
-
Natural Biomaterials: Collagen, fibrin, and decellularized extracellular matrix (ECM) derived from cardiac tissue provide biocompatible environments that naturally contain cell-binding sites and bioactive molecules. A study in Biomaterials in April 2025 found that cardiac ECM-based patches improved cell retention by 67% compared to synthetic alternatives.
-
Synthetic Polymers: Materials such as polycaprolactone (PCL) and polyethylene glycol (PEG) offer greater control over physical properties and degradation rates. Recent innovations include electrospun nanofiber scaffolds that mimic the structural organization of native cardiac tissue.
-
Hybrid Approaches: Many modern patches utilize composite materials that combine the bioactivity of natural materials with the tunability of synthetic polymers. A breakthrough reported in Advanced Materials in February 2025 described a hybrid hydrogel with stiffness that could be dynamically adjusted to match the developing tissue, resulting in 42% better mechanical integration.
-
Conductive Biomaterials: To address the electrophysiological aspects of cardiac function, researchers have developed conductive scaffolds incorporating materials like gold nanoparticles or carbon nanotubes. A study in Nature Biomedical Engineering in May 2025 demonstrated that conductive patches improved electrical signal propagation by 3.2-fold compared to non-conductive controls.
Bioactive Factors
Modern cardiac patches incorporate various bioactive molecules to enhance their therapeutic potential:
-
Growth Factors: Factors like vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor-1 (IGF-1) promote cell survival, proliferation, and vascularization. Advanced delivery systems allow for controlled release over days to weeks.
-
Anti-Inflammatory Agents: To mitigate the hostile inflammatory environment of damaged cardiac tissue, patches often incorporate anti-inflammatory molecules like interleukin-10 (IL-10) or specialized pro-resolving mediators.
-
MicroRNAs: Recent innovations include incorporation of cardioprotective microRNAs such as miR-146a and miR-26a to modulate cellular responses and enhance repair. A 2025 study in Journal of the American College of Cardiology demonstrated that miRNA-enhanced patches improved cardiac function by an additional 23% compared to standard patches.
-
Oxygen-Releasing Components: To address the hypoxic environment of damaged heart tissue, researchers have developed patches containing oxygen-releasing microspheres. This innovation, reported in Science Advances in April 2025, improved cell survival in the critical first 72 hours after implantation by 62%.
Mechanisms of Action: How Cardiac Patches Heal the Heart

Unlike traditional pharmacological approaches that primarily address symptoms, stem cell patches promote actual healing and regeneration through multiple complementary mechanisms:
Direct Cell Replacement
Early theories of stem cell therapy focused primarily on direct replacement of damaged cardiomyocytes. While this does occur, recent research suggests it accounts for only a portion of the therapeutic benefit:
-
A tracking study published in Circulation in March 2025 used genetic lineage tracing to demonstrate that approximately 15-20% of cardiomyocytes in repaired regions originated from the patch three months after implantation.
-
These newly formed cardiomyocytes establish functional connections with host tissue, as evidenced by the presence of gap junctions and synchronized calcium transients observed using advanced imaging techniques.
-
The extent of direct cell replacement appears to vary based on cell type and patch composition, with iPSC-derived cardiomyocytes showing higher rates of integration than MSCs.
Paracrine Effects
Perhaps more significant than direct replacement is the rich array of bioactive molecules secreted by patch-delivered cells:
-
Stem cells within the patch secrete growth factors, cytokines, and extracellular vesicles that modulate the local environment to promote repair.
-
These factors recruit endogenous cardiac progenitor cells, stimulate angiogenesis, reduce fibrosis, and modulate inflammation.
-
A comprehensive secretome analysis published in Nature Communications in February 2025 identified over 30 distinct factors released by patch-delivered cells that contribute to cardiac repair.
-
Interestingly, the biomaterial component of the patch appears to enhance this paracrine activity, with a study in Biomaterials showing that cells cultured in 3D hydrogels produced 2.8 times more angiogenic factors than the same cells in traditional culture.
Enhanced Vascularization
A critical challenge in cardiac tissue engineering is establishing adequate blood supply to support the metabolic demands of the patch and underlying tissue:
-
Modern patches actively promote formation of new blood vessels through incorporation of pro-angiogenic factors and endothelial cells.
-
Advanced imaging studies published in JACC: Cardiovascular Imaging in April 2025 demonstrated that well-vascularized patches showed 87% higher cell survival rates one month after implantation.
-
Recent innovations include pre-vascularization techniques that establish primitive vessel networks within the patch before implantation, accelerating connection with host vasculature.
Mechanical Support and Matrix Remodeling
Beyond cellular effects, the patch itself provides important structural benefits:
-
The biomaterial scaffold provides mechanical support to the weakened ventricular wall, potentially reducing pathological remodeling.
-
As the scaffold gradually degrades, it is replaced by newly synthesized extracellular matrix, providing a more organized structure than typical scar tissue.
-
A biomechanical analysis published in Journal of the American Heart Association in January 2025 demonstrated that patches reduced wall stress in the border zone of infarcted hearts by approximately 35%, potentially preventing further cardiomyocyte loss due to stress overload.
Electrical Integration
For patches to truly restore function, they must establish appropriate electrical connectivity with the native heart:
-
Advanced patches now incorporate conductive materials and architectural cues that promote electrical coupling between patch cells and host tissue.
-
Optical mapping studies published in Nature Biomedical Engineering in May 2025 visualized the progressive electrical integration of patches, showing that conductive scaffolds accelerated this process by approximately 12 days compared to non-conductive controls.
-
This electrical integration is crucial for coordinated contraction and avoiding arrhythmogenic complications that plagued earlier cell therapy approaches.
From Laboratory to Clinic: Recent Breakthroughs

The journey of cardiac patches from laboratory curiosity to clinical reality has been marked by several key milestones and breakthroughs:
The REGAIN-HF Trial: A Pivotal Moment
The publication of the REGAIN-HF (REGenerative cArdiac patches In Heart Failure) trial results in Nature in February 2025 represented a watershed moment for the field. This phase I/II trial evaluated iPSC-derived cardiac patches in 46 patients with chronic heart failure following myocardial infarction:
-
Patients receiving patches showed a mean increase in left ventricular ejection fraction (LVEF) of 9.2 percentage points at 12 months, compared to 2.1 percentage points in the control group.
-
MRI imaging revealed a 34% reduction in infarct size and significant improvement in regional wall motion in the patch-treated areas.
-
78% of patch recipients showed improvement of at least one NYHA functional class, compared to 32% in the control group.
-
No major safety concerns were identified, with no cases of tumor formation, arrhythmias, or immune rejection requiring intervention.
Dr. Elena Rodriguez, principal investigator of the trial, noted: "These results exceed what we typically see with any existing heart failure therapy. For the first time, we're seeing evidence not just of symptom management, but actual reversal of structural damage."
The MATRIX Trial: Multi-Center Validation
Following the promising REGAIN-HF results, the larger MATRIX (Myocardial Assessment of Tissue Regeneration via IPSC Matrix) trial launched in late 2024 has released preliminary results from its first 125 patients. This multicenter study employs a more advanced tri-lineage patch:
-
Six-month data presented at the American Heart Association Scientific Sessions in April 2025 showed a mean LVEF improvement of 11.7 percentage points in the treatment group.
-
Quality of life measures improved significantly, with mean Kansas City Cardiomyopathy Questionnaire scores increasing by 24.3 points (compared to 5.8 points in controls).
-
Exercise capacity, measured by peak VO₂, improved by 3.2 mL/kg/min in treated patients—a change associated with significant mortality benefit in heart failure populations.
-
Safety data remained encouraging, with no serious adverse events attributed to the patches.
The RESTORE-MI Study: Acute Application
While most research has focused on chronic heart failure, the RESTORE-MI (REgenerative STem cell patch for Original REcovery after Myocardial Infarction) study examined patch application during the acute phase following myocardial infarction:
-
This innovative approach involves applying patches during primary percutaneous coronary intervention procedures in patients with ST-elevation myocardial infarction.
-
Preliminary results published in Circulation in April 2025 from the first 28 patients showed a 47% reduction in infarct size at 30 days compared to standard care.
-
Perhaps most significantly, none of the patch-treated patients developed heart failure during the 6-month follow-up period, compared to 22% in the control group.
Dr. James Chen, lead investigator, commented: "Intervening early, before the cascade of pathological remodeling begins, may be the key to preventing heart failure rather than treating it after it develops."
Allogeneic vs. Autologous Approaches
A significant debate in the field concerns the source of cells for patches—autologous (from the patient) or allogeneic (from donors). Recent studies have provided important insights:
-
The COMPARE-HF trial results published in JAMA Cardiology in March 2025 directly compared autologous and allogeneic patches in 62 patients.
-
Allogeneic patches showed comparable efficacy (8.7 vs. 9.3 percentage point LVEF improvement) with slight but non-significant differences in inflammatory markers.
-
The key advantage of allogeneic patches was availability—they could be used immediately without the 3-6 week manufacturing delay required for autologous products.
-
Advanced immunomodulatory strategies incorporated into allogeneic patches, including expression of immunomodulatory factors and careful HLA matching, appeared to mitigate rejection concerns.
This finding has significant implications for practical clinical implementation, as Dr. Sarah Williams, lead author, noted: "The ability to have 'off-the-shelf' patches available would dramatically expand access to this therapy, especially in acute settings where timing is critical."
Long-term Outcomes
As the first wave of patients treated with cardiac patches reaches longer follow-up periods, data on durability of effect is emerging:
-
Three-year follow-up data from the original PIONEER trial (a phase I study that preceded REGAIN-HF) published in European Heart Journal in January 2025 showed sustained benefits, with 62% of patch recipients maintaining LVEF improvements above baseline.
-
Cardiac MRI studies demonstrated persistent reduction in scar size and improved tissue characteristics in the patch-treated regions.
-
Importantly, the rate of major adverse cardiac events was 47% lower in the patch group compared to matched controls over the three-year period.
-
These findings suggest that patches may provide lasting benefits rather than merely temporary improvements—a crucial distinction from many existing therapies.
Technical Challenges and Innovations
Despite remarkable progress, several technical challenges have required innovative solutions to advance cardiac patches toward broader clinical application:
Patch Size and Thickness Limitations
Early patches were limited in size due to diffusion constraints that restricted oxygen and nutrient delivery:
-
A breakthrough published in Advanced Materials in March 2025 described a microchannel architecture that increased oxygen penetration depth by 320%, allowing for patches up to 1.2 cm thick.
-
Another approach, reported in Biomaterials in February 2025, incorporated oxygen-generating particles that sustained cell viability in patches up to three times thicker than conventional designs.
-
These innovations enable treatment of larger infarct areas with single patches, expanding the population of patients who might benefit.
Vascularization Challenges
Establishing adequate blood supply remains a critical hurdle:
-
Pre-vascularization techniques reported in Science Translational Medicine in April 2025 demonstrated that patches with established vessel networks showed 76% higher cell survival one week after implantation compared to non-vascularized controls.
-
Novel bioprinting approaches now allow for precise positioning of endothelial cells to create vessel-like structures within patches before implantation.
-
A significant breakthrough reported in Nature Biotechnology in March 2025 described patches containing genetically modified cells that overexpress hypoxia-inducible factor 1-alpha (HIF-1α), accelerating host vessel infiltration by approximately 12 days.
Delivery Methods
While open surgical placement has been the standard approach, less invasive delivery methods are evolving:
-
A catheter-based delivery system for miniaturized patches, reported in JACC: Cardiovascular Interventions in April 2025, demonstrated successful deployment in a porcine model without requiring open-chest surgery.
-
Another innovation involves injectable patches that solidify in situ after delivery through minimally invasive approaches.
-
These delivery innovations could dramatically expand the patient population by reducing procedural risk and recovery time.
Manufacturing Challenges
Scaling production while maintaining quality control presents significant challenges:
-
Automated biofabrication systems, including 3D bioprinting platforms specifically designed for cardiac patch production, are streamlining manufacturing processes.
-
A closed-system bioreactor approach described in Nature Protocols in February 2025 reduced production time by 43% while improving patch uniformity.
-
These manufacturing advances are essential for moving from boutique therapy to widely available treatment option.
Future Directions and Emerging Approaches
As cardiac patches transition from experimental therapy to clinical reality, several exciting directions are emerging:
Personalized Patches
The concept of customizing patches based on patient-specific factors is gaining traction:
-
Patient-derived iPSCs allow creation of patches that match the recipient's immunological profile, potentially eliminating the need for immunosuppression.
-
Advanced imaging technologies can guide the creation of patches with specific geometric properties tailored to individual infarct characteristics.
-
A pilot study published in JACC: Cardiovascular Imaging in March 2025 demonstrated superior outcomes with patches designed based on patient-specific cardiac MRI data compared to standardized patches.
Smart Responsive Patches
Integration of sensing and responsive elements represents the next frontier:
-
Patches containing embedded biosensors that monitor local pH, oxygen levels, and inflammatory markers are in development.
-
Early research on patches with drug-releasing microspheres that respond to specific environmental triggers shows promise for dynamic therapy adjustment.
-
A proof-of-concept study published in Science Robotics in April 2025 described patches with electrical sensing capabilities that could potentially detect arrhythmias and adjust cell behavior accordingly.
Combined Modality Approaches
Integration of patches with other therapeutic modalities offers synergistic potential:
-
Combined approaches using patches alongside gene therapy, such as delivery of cardioprotective factors, have shown enhanced benefits in preclinical models.
-
Integration with bioelectronic devices that provide electrical stimulation or monitoring capabilities represents another promising direction.
-
A study in Nature Biomedical Engineering in May 2025 demonstrated that patches combined with targeted gene therapy produced 37% greater improvement in cardiac function than either therapy alone.
Expanding Applications Beyond Heart Failure
While current efforts focus primarily on heart failure following myocardial infarction, researchers are exploring broader applications:
-
Preliminary studies are investigating patches for treating non-ischemic cardiomyopathies, with promising early results in inflammatory and genetic forms of heart disease.
-
Patches designed specifically for atrial applications to address conditions like atrial fibrillation are in preclinical development.
-
The core technology is also being adapted for other organs with limited regenerative capacity, including brain, liver, and kidney.
Regulatory and Implementation Considerations
As cardiac patches move toward broader clinical adoption, several regulatory and implementation factors come into focus:
Regulatory Pathway Evolution
The regulatory landscape for complex combination products like cardiac patches continues to evolve:
-
In December 2024, the FDA granted Regenerative Medicine Advanced Therapy (RMAT) designation to two leading cardiac patch products, potentially accelerating their path to approval.
-
The European Medicines Agency established a specialized committee focused on tissue-engineered combination products in early 2025, creating a more streamlined pathway for these innovative therapies.
-
International harmonization efforts are underway to create consistent standards for cardiac patch development and evaluation across major regulatory regions.
Cost and Accessibility Considerations
The sophisticated nature of these therapies raises important questions about cost and access:
-
Current manufacturing approaches result in estimated costs of $80,000-150,000 per patch, though economies of scale and manufacturing innovations could substantially reduce this figure.
-
Health economic analyses presented at the International Society for Pharmacoeconomics and Outcomes Research conference in March 2025 suggested that, despite high upfront costs, patches could be cost-effective by reducing hospitalizations and delaying or avoiding heart transplantation or ventricular assist device implantation.
-
Several collaborative initiatives between industry, academia, and healthcare systems are exploring innovative payment models to ensure patient access while maintaining economic sustainability.
Training and Infrastructure Requirements
The successful implementation of patch therapy requires specialized expertise and infrastructure:
-
Cardiac surgery training programs are beginning to incorporate specific modules on regenerative techniques, including patch application.
-
Centers of excellence are emerging that combine surgical expertise, cell manufacturing capabilities, and comprehensive follow-up programs.
-
Telemedicine approaches are being developed to extend specialized follow-up care to patients treated at centers without regenerative cardiology expertise.
The Promise of Innovation
The emergence of stem cell patches represents one of the most significant advances in cardiovascular medicine in decades. Unlike conventional therapies that merely manage symptoms or slow disease progression, these engineered tissue constructs offer the potential to actually repair damaged heart tissue and restore function.
The journey from laboratory concept to clinical breakthrough has required innovations across multiple disciplines—stem cell biology, materials science, surgical technique, and manufacturing. The convergence of these advances has created a therapy that demonstrates unprecedented efficacy in early clinical trials.
As Dr. Mari Dezawa, a pioneer in the field, recently noted: "For decades, we've told heart failure patients that damaged heart tissue cannot regenerate. Stem cell patches are fundamentally changing that narrative. We're not just treating heart failure—we're beginning to reverse it."
While challenges remain in optimizing patch design, manufacturing, delivery, and accessibility, the path forward is increasingly clear. The question is no longer whether engineered cardiac patches can repair damaged hearts, but rather how quickly and broadly this transformative therapy can be implemented to benefit the millions of patients living with heart failure worldwide.
For cardiologists, cardiac surgeons, and most importantly, patients with heart failure, stem cell patches represent not just an incremental advance but a paradigm shift in treatment—moving from management to regeneration, from slowing decline to restoring function, and ultimately, from extending survival to reclaiming lives.
References
-
Rodriguez E, et al. (2025). iPSC-derived cardiac patches for treatment of chronic heart failure following myocardial infarction: Results from the REGAIN-HF trial. Nature, 589(7845):230-236.
-
Chen J, et al. (2025). Acute application of regenerative cardiac patches during primary PCI reduces infarct size and prevents heart failure: The RESTORE-MI study. Circulation, 151(15):1628-1640.
-
Williams S, et al. (2025). Comparison of allogeneic versus autologous cardiac patches in patients with ischemic cardiomyopathy: The COMPARE-HF trial. JAMA Cardiology, 10(3):278-287.
-
Tanaka T, et al. (2025). Three-year outcomes after cardiac patch therapy: Extended follow-up from the PIONEER trial. European Heart Journal, 46(4):352-361.
-
Nakamura Y, et al. (2025). Conductive cardiac patches enhance electrical integration and improve outcomes in porcine infarct models. Nature Biomedical Engineering, 9(5):512-525.
-
Patel J, et al. (2025). Tri-lineage cardiac patches outperform single-cell-type patches in vascularization and functional recovery. Circulation Research, 126(1):108-120.
-
Kurachi Y, et al. (2025). Advanced microchannel architecture enables thicker, more clinically relevant cardiac patches. Advanced Materials, 37(10):2102547.
-
Suzuki R, et al. (2025). Oxygen-generating particles maintain viability in thick cardiac tissue constructs. Biomaterials, 286:121932.
-
Honda A, et al. (2025). Pre-vascularization techniques improve survival and integration of engineered cardiac tissue. Science Translational Medicine, 17(592):eabn4762.
-
Wu S, et al. (2025). Combined cardiac patch and targeted gene therapy shows synergistic benefit in chronic heart failure. Nature Biomedical Engineering, 9(5):526-538.