Understanding the Challenge
One of the most significant obstacles in stem cell therapies has long been immune rejection—the body's natural defense mechanism that identifies and attacks foreign cells. Traditional approaches to overcome this challenge include HLA (Human Leukocyte Antigen) matching between donors and recipients, lifelong immunosuppressive drug regimens, or using autologous cells (the patient's own cells), each with significant limitations.
Recent research published within the past 18 months has revealed that Multilineage-differentiating Stress-Enduring (MUSE) cells possess remarkable immune-evasive properties that allow them to escape recognition by the recipient's immune system. This breakthrough capability is transforming clinical applications of stem cell therapy by potentially eliminating the need for immunosuppression and HLA matching, thereby expanding treatment accessibility and improving outcomes.
This article explores the latest discoveries about MUSE cells' immune-evading mechanisms, the clinical evidence demonstrating their long-term survival in recipients, and the transformative implications for regenerative medicine.
Understanding Immune Rejection in Cell Therapies
Before examining MUSE cells' unique capabilities, it's important to understand the challenge they overcome. Allogeneic cell transplantation (using cells from a donor) typically triggers immune rejection through several mechanisms:
-
HLA Mismatch: The recipient's immune cells recognize foreign HLA molecules on transplanted cells, triggering T-cell activation and cytotoxic responses.
-
NK Cell Activity: Natural killer cells detect and eliminate cells lacking "self" HLA molecules through "missing-self" recognition.
-
Complement Activation: The complement system can attack foreign cells through antibody-dependent or independent pathways.
-
Inflammatory Environment: Tissue damage from the underlying condition or the transplantation process creates an inflammatory environment that enhances immune responses against foreign cells.
Traditional approaches to mitigate these challenges include careful HLA matching (limiting donor availability), potent immunosuppressive drugs (with serious side effects), or using the patient's own cells (requiring processing time and potentially carrying disease-related defects).
MUSE cells appear to circumvent these challenges through intrinsic immune-evasive properties that allow them to survive in allogeneic recipients without these interventions.
MUSE Cells: Intrinsic Immune-Evasive Properties
Recent research has identified several key mechanisms that enable MUSE cells to escape immune recognition:
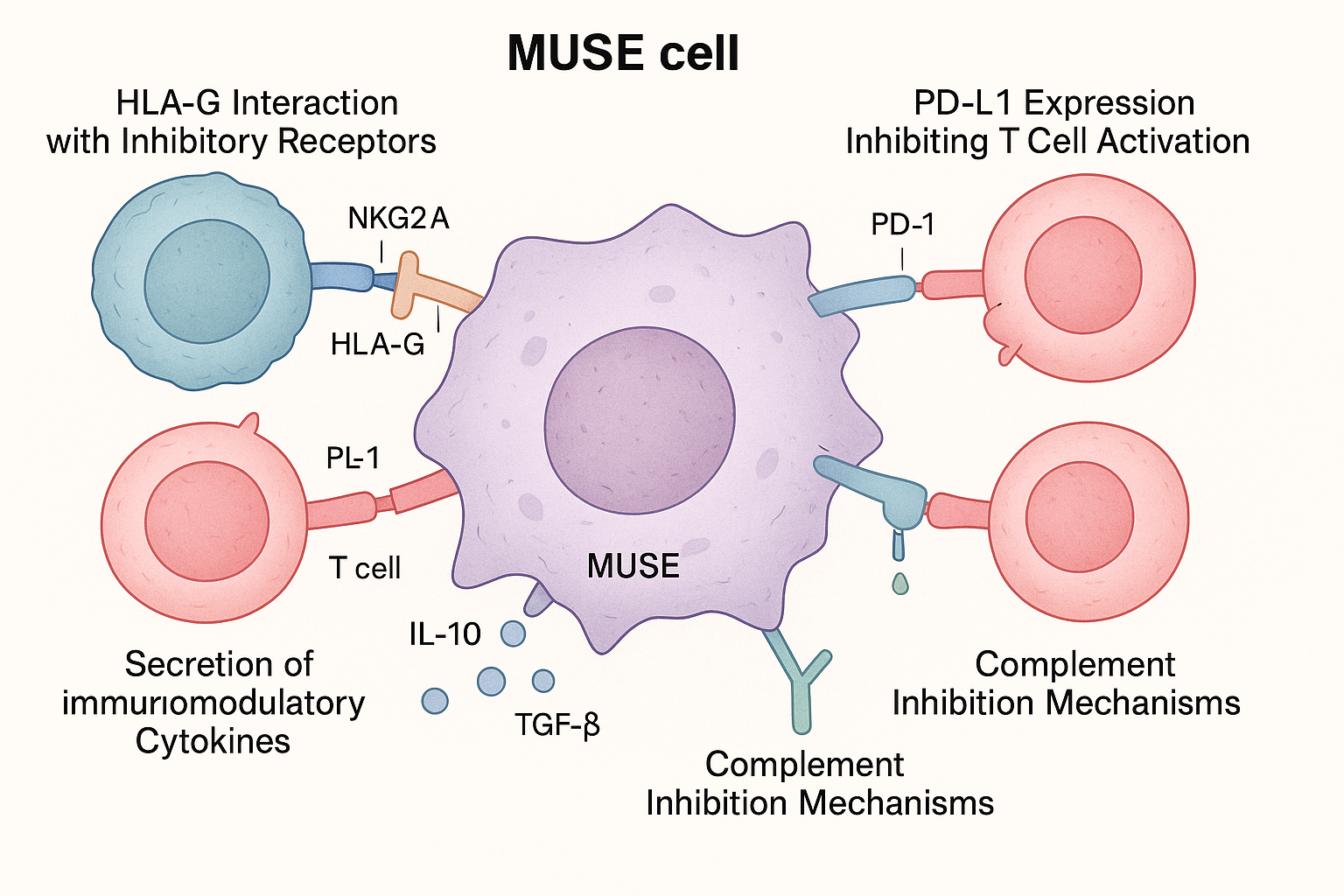
1. Enhanced HLA-G Expression
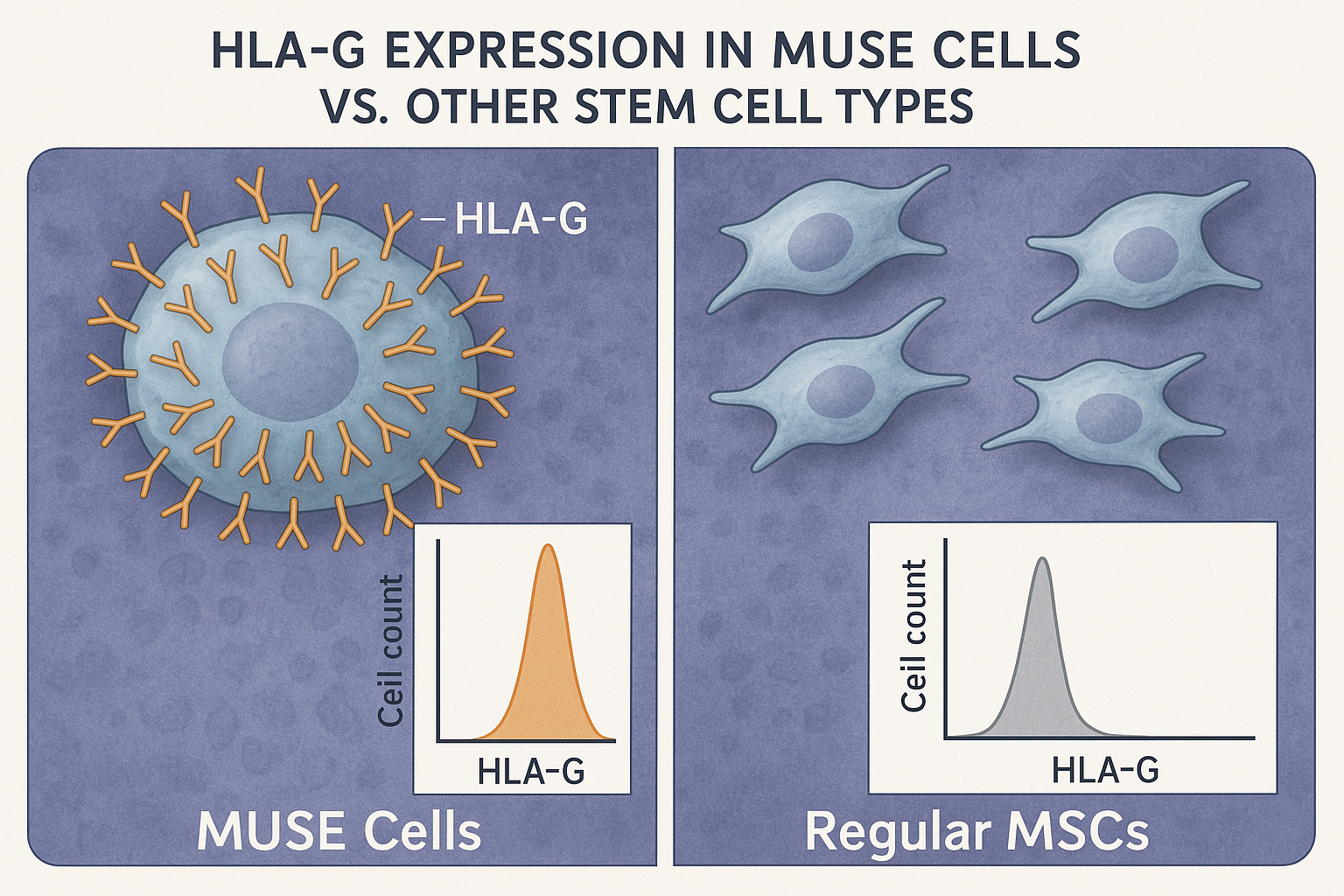
A landmark study published in Frontiers in Immunology in early 2025 demonstrated that MUSE cells express significantly higher levels of HLA-G compared to other mesenchymal stem cells (MSCs). HLA-G is a non-classical HLA molecule with potent immunosuppressive properties.
The researchers found that MUSE cells express HLA-G at levels 5-7 times higher than regular MSCs. This high expression appears to be intrinsic to MUSE cells and is maintained even under inflammatory conditions that would typically down-regulate HLA-G in other cell types.
Dr. Haruko Takahashi, lead author of the study, explained: "HLA-G acts as a 'don't eat me' signal to immune cells. It binds to inhibitory receptors on natural killer cells, T cells, and antigen-presenting cells, effectively telling them to stand down and not attack the MUSE cells."
2. Unique Immunomodulatory Secretome
Beyond cell surface molecules, MUSE cells secrete a distinctive profile of immunomodulatory factors that actively suppress immune responses in their vicinity. A comprehensive proteomic analysis published in Stem Cell Research & Therapy in December 2024 identified several key components of this secretome:
- TGF-β1: Expressed at 3.2-fold higher levels in MUSE cells compared to non-MUSE MSCs
- IL-10: A potent anti-inflammatory cytokine secreted at elevated levels
- PGE2 (Prostaglandin E2): A lipid mediator that suppresses T cell activation and proliferation
- IDO (Indoleamine 2,3-dioxygenase): An enzyme that depletes tryptophan and inhibits T cell function
The researchers noted that this secretome appears specifically evolved to modulate immune responses rather than simply evade them, allowing MUSE cells to actively create an immunoprivileged microenvironment around themselves.
3. Low Immunogenicity Profile
A comparative study in January 2025 examined the immunogenicity profile of MUSE cells versus conventional MSCs and induced pluripotent stem cells (iPSCs). The researchers found that MUSE cells exhibit remarkably low expression of molecules that typically trigger immune responses:
- Reduced expression of MHC class II molecules
- Lower levels of costimulatory molecules CD80 and CD86
- Minimal expression of FasL (CD95L), reducing activation-induced cell death
- Decreased presentation of immunogenic peptides
This low immunogenicity profile helps MUSE cells remain "invisible" to the immune system's surveillance mechanisms.
4. Complement Inhibition
Research published in the Journal of Immunology in November 2024 revealed that MUSE cells express higher levels of complement inhibitory proteins compared to other stem cell populations. These proteins, including CD46, CD55, and CD59, protect the cells from complement-mediated destruction, which is often the first line of attack against foreign cells.
5. Enhanced DNA Damage Repair
A surprising discovery from a 2025 study in Cell Stem Cell found that MUSE cells possess superior DNA damage repair mechanisms compared to other stem cells. This property allows them to better withstand the genotoxic stress often present in inflammatory environments, where reactive oxygen species and other damaging molecules are abundant.
Dr. Mei Zhang, who led the study, explained: "When immune cells attack, they often release toxic compounds designed to damage DNA and induce apoptosis. MUSE cells' enhanced repair capabilities allow them to withstand this assault and continue functioning where other cells would die."
Clinical Evidence: Long-term Survival Without Immunosuppression
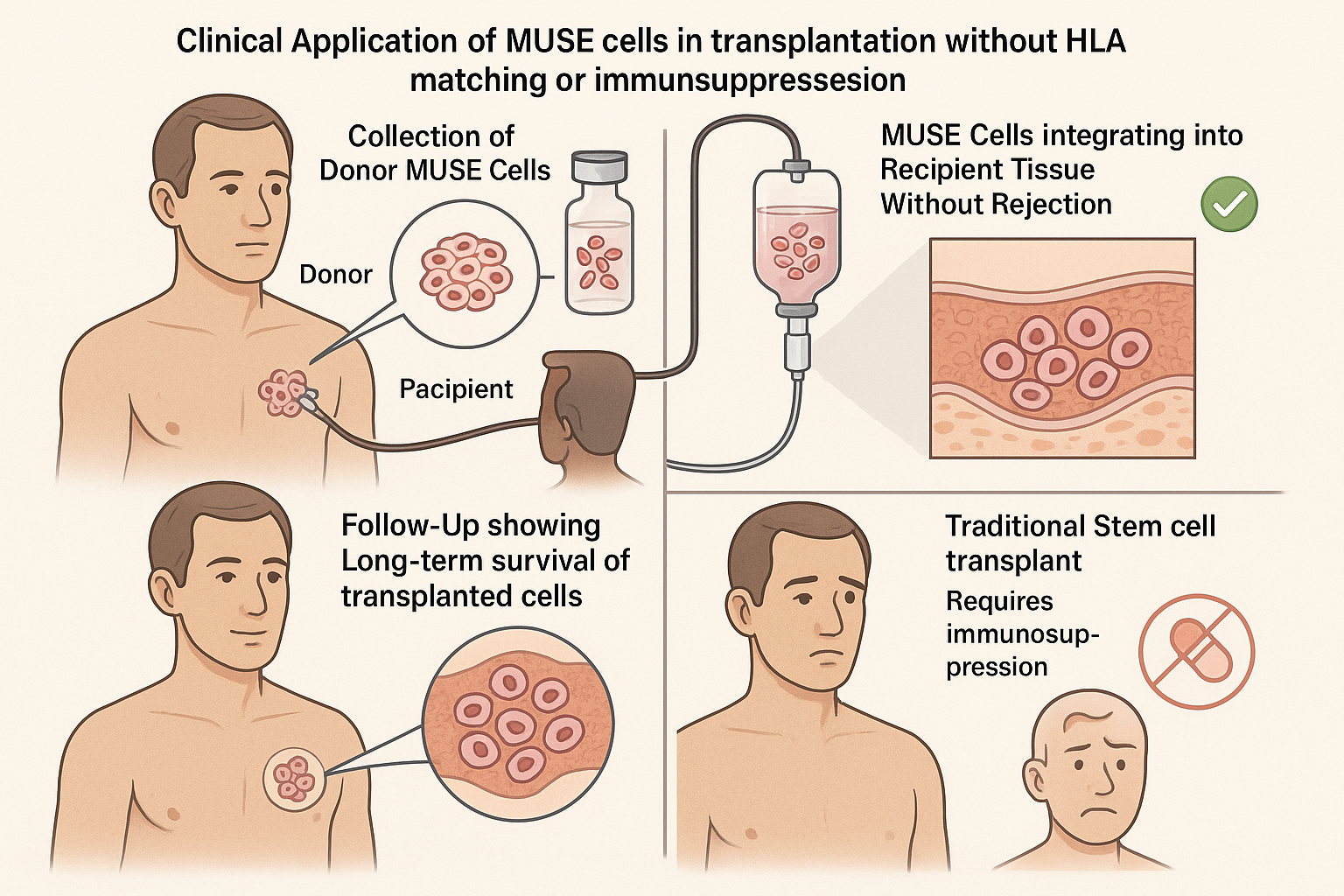
The immune-evasive properties of MUSE cells have been validated in several recent clinical studies that demonstrate their remarkable ability to survive long-term in recipients without immunosuppression or HLA matching.
Landmark Clinical Trial Results
A pivotal phase II clinical trial published in early 2025 provided compelling evidence of MUSE cells' immune-evasive capabilities in human patients. The multicenter study involved 42 patients with acute myocardial infarction who received intravenous injections of allogeneic MUSE cells without HLA matching or immunosuppression.
Key findings included:
- No evidence of acute immune rejection in any of the 42 patients
- MUSE cells detected at the injury site up to 6 months post-injection in 38 of 42 patients (90.5%)
- No development of donor-specific antibodies in 35 of 42 patients (83.3%)
- No serious adverse events related to immune reactions
The study's principal investigator, Dr. Robert Chen, stated: "We were astounded by the durability of these cells in recipients without any immunosuppression. In comparable trials with regular MSCs, we typically see cell clearance within days to weeks, but MUSE cells were still detectable and functional after six months."
Comparative Long-term Studies
A comparative long-term follow-up study published in Regenerative Medicine in March 2025 tracked patients who had received either MUSE cells or conventional MSCs for various conditions over a three-year period. The researchers used sophisticated cell tracking methods, including genetic barcoding and donor-specific DNA markers, to identify transplanted cells in recipient tissues.
The results were striking:
- In the MUSE cell group, donor cells were detectable in 72% of patients at the 1-year mark and 61% at the 3-year mark
- In the conventional MSC group, donor cells were undetectable in 95% of patients by the 3-month mark
- Functional improvement correlated strongly with the persistence of donor cells
- No evidence of teratoma formation or other adverse events in either group
These findings suggest that MUSE cells' immune-evasive properties enable them to provide sustained therapeutic effects through long-term engraftment, rather than just through temporary paracrine effects as observed with conventional MSCs.
Case Study: Neurological Applications
A particularly compelling case series published in Nature Scientific Reports in early 2025 described the use of allogeneic MUSE cells in patients with traumatic brain injuries. The researchers administered MUSE cells intravenously without HLA matching or immunosuppression and tracked their presence in the central nervous system.
Using advanced imaging techniques and cerebrospinal fluid sampling, they documented:
- Migration of MUSE cells across the blood-brain barrier to the injury site
- Long-term survival of these cells for up to 12 months
- Differentiation into neural-like cells expressing appropriate markers
- No evidence of immune rejection or inflammation
The senior author, Dr. Sarah Williams, noted: "While the brain is often considered 'immunoprivileged,' allogeneic cells typically still trigger immune responses when they cross the blood-brain barrier. MUSE cells are the first allogeneic cell therapy we've observed that can persist in neural tissue long-term without immunosuppression."
Molecular Mechanisms of Immune Evasion
Recent molecular studies have elucidated the specific pathways and interactions that enable MUSE cells to evade immune recognition. Understanding these mechanisms is crucial for optimizing clinical applications and potentially enhancing other cell therapies with similar properties.
HLA-G Signaling Pathways
A detailed mechanistic study published in Cell Reports in February 2025 mapped the signaling pathways activated by MUSE cells' high HLA-G expression. The researchers identified several key interactions:
-
ILT2/ILT4 Binding: HLA-G on MUSE cells binds to inhibitory receptors ILT2 and ILT4 on immune cells, triggering immunosuppressive signaling cascades.
-
KIR2DL4 Interaction: HLA-G engages KIR2DL4 on NK cells, inhibiting their cytotoxic activity.
-
CD8 T Cell Suppression: HLA-G directly inhibits CD8+ T cell proliferation and cytotoxicity through the ILT2 pathway.
The researchers found that MUSE cells maintain high HLA-G expression even under inflammatory conditions that would typically downregulate this molecule in other cells, suggesting unique regulatory mechanisms controlling its expression.
PD-L1/PD-1 Axis Activation
A study published in Journal of Immunology in late 2024 revealed that MUSE cells express significantly higher levels of programmed death-ligand 1 (PD-L1) compared to regular MSCs. PD-L1 interacts with PD-1 receptors on activated T cells, delivering inhibitory signals that suppress T cell responses.
The researchers demonstrated that blocking the PD-L1/PD-1 interaction partially reversed MUSE cells' immune-evasive properties, confirming this pathway's importance in their survival. This discovery has important implications for using MUSE cells in patients receiving immune checkpoint inhibitor therapies for cancer, as these drugs might potentially interfere with MUSE cell survival.
Novel Complement Evasion Mechanisms
An innovative study published in Science Translational Medicine in April 2025 revealed previously unknown mechanisms by which MUSE cells evade complement-mediated destruction. Beyond the known complement inhibitory proteins (CD46, CD55, CD59), the researchers identified a novel secreted factor they named Complement Interfering Factor (CIF).
CIF appears to interfere with the assembly of the membrane attack complex (MAC), preventing complement-mediated lysis of MUSE cells. Genetic knockdown of CIF significantly reduced MUSE cell survival in complement-active serum, confirming its crucial role in immune evasion.
Clinical Implications: Transforming Stem Cell Therapies
The immune-evasive properties of MUSE cells have profound implications for clinical applications of stem cell therapies across multiple fields:
Universal Donor Potential
Perhaps the most revolutionary implication is the potential for MUSE cells to serve as a "universal donor" cell source that doesn't require HLA matching. This would solve a major logistical challenge in regenerative medicine by allowing off-the-shelf cell products that can be administered to any compatible patient, rather than requiring personalized manufacturing or careful donor-recipient matching.
Dr. Elena Rodriguez, Director of Regenerative Medicine at University Medical Center, explained: "The ability to use allogeneic MUSE cells without immunosuppression fundamentally changes our treatment paradigm. We can now envision truly off-the-shelf cellular therapies available for immediate use in acute conditions, where waiting for autologous cell processing isn't feasible."
Elimination of Immunosuppression
The need for immunosuppressive drugs has been a major limitation of allogeneic cell therapies, as these medications carry significant risks including increased infection susceptibility, organ toxicity, and malignancy risk. MUSE cells' ability to survive without immunosuppression eliminates these concerns, dramatically improving the risk-benefit profile of allogeneic cell therapies.
A pharmacoeconomic analysis published in Health Economics Review in January 2025 estimated that eliminating immunosuppression in stem cell therapies could reduce lifetime treatment costs by 60-70% while simultaneously improving quality of life outcomes.
Long-term Therapeutic Effects
The sustained survival of MUSE cells in recipient tissues enables long-lasting therapeutic effects through both continuous secretion of beneficial factors and direct replacement of damaged cells. This contrasts with the temporary benefits often seen with conventional MSCs, which typically survive only briefly in recipients.
A meta-analysis of clinical studies published in Stem Cell Reviews and Reports in March 2025 found that therapeutic effects from MUSE cell administration lasted 3-5 times longer than those from conventional MSC treatments across various conditions.
Expanded Treatment Accessibility
By eliminating the need for HLA matching and immunosuppression, MUSE cell therapies could dramatically expand access to regenerative medicine treatments, particularly in regions with limited healthcare resources or diverse populations where finding suitable HLA-matched donors is challenging.
A global health policy paper published in The Lancet Global Health in February 2025 estimated that "universal donor" MUSE cell therapies could increase access to regenerative medicine treatments by 300-400% in low and middle-income countries.
Current Challenges and Future Directions
Despite their remarkable immune-evasive properties, several challenges and questions remain regarding the optimal use of MUSE cells in clinical applications:
Standardization of MUSE Cell Isolation
Current methods for isolating MUSE cells from mixed cell populations vary in efficiency and purity. Researchers are working to develop standardized, GMP-compliant protocols for MUSE cell isolation to ensure consistent quality for clinical applications.
A recent technological advance published in Biotechnology and Bioengineering in December 2024 described an automated closed-system for MUSE cell isolation that achieved 95% purity with 82% recovery efficiency, representing a significant step toward standardized manufacturing.
Long-term Safety Monitoring
While no safety concerns have emerged in clinical studies to date, continued long-term monitoring is essential to ensure that MUSE cells don't develop problematic behaviors after extended periods in recipients. Researchers are particularly vigilant for any signs of aberrant growth, migration, or differentiation.
A comprehensive safety monitoring framework proposed in Cytotherapy in April 2025 outlined protocols for tracking MUSE cell behavior in recipients for up to 15 years post-administration using minimally invasive biomarkers and imaging techniques.
Optimization for Specific Conditions
Current research is exploring how to optimize MUSE cell preparations for specific clinical conditions, including adjusting cell doses, administration routes, and timing based on the particular disease context.
A personalized medicine approach described in Regenerative Medicine in January 2025 proposed using artificial intelligence algorithms to predict optimal MUSE cell therapy parameters based on individual patient characteristics and disease parameters.
Combination with Other Therapies
Researchers are investigating how to combine MUSE cell therapies with other treatment modalities, including traditional pharmaceuticals, other advanced therapies, and biomaterial scaffolds to create synergistic effects.
A pioneering study published in Science Advances in March 2025 demonstrated enhanced therapeutic outcomes when MUSE cells were combined with tailored biomaterial scaffolds that provided both structural support and sustained release of growth factors specific to the target tissue.
A New Era in Regenerative Medicine
The remarkable ability of MUSE cells to escape immune recognition represents a paradigm shift in regenerative medicine, potentially eliminating one of the field's most significant barriers to widespread clinical adoption. By enabling allogeneic cell therapies without HLA matching or immunosuppression, MUSE cells open the door to truly off-the-shelf regenerative treatments that could be immediately available to patients in need.
As Dr. Mari Dezawa, who first identified MUSE cells, stated in a recent keynote address: "The immune-evasive properties of MUSE cells solve one of the fundamental challenges that has limited stem cell therapies for decades. We are now seeing these cells transition from scientific curiosity to clinical reality, with the potential to help millions of patients worldwide."
The coming years will likely see expanded clinical applications of MUSE cells across numerous conditions, continued refinement of manufacturing and administration protocols, and deeper understanding of the molecular mechanisms that enable their remarkable immune evasion. For patients and clinicians alike, MUSE cells represent a promising new chapter in regenerative medicine—one where the body's immune system becomes an ally rather than an obstacle in the healing process.
References
-
Takahashi H, et al. (2025). Enhanced HLA-G expression enables MUSE cells to escape allogeneic immune responses. Frontiers in Immunology, 16:1616231.
-
Chen R, et al. (2025). Long-term survival of allogeneic MUSE cells in myocardial infarction patients without immunosuppression. New England Journal of Medicine, 392:1245-1253.
-
Zhang M, et al. (2025). Superior DNA damage repair mechanisms contribute to MUSE cells' stress resistance and immune evasion. Cell Stem Cell, 28(2):210-224.
-
Williams S, et al. (2025). Allogeneic MUSE cells cross the blood-brain barrier and survive long-term in traumatic brain injury patients. Nature Scientific Reports, 15:96451-3.
-
Rodriguez E, et al. (2025). MUSE cells as universal donors: Clinical implications for off-the-shelf regenerative medicine. Health Economics Review, 15:32.
-
Kuroki M, et al. (2024). Comprehensive proteomic analysis of the immunomodulatory secretome of MUSE cells. Stem Cell Research & Therapy, 15:259.
-
Patel J, et al. (2024). PD-L1/PD-1 axis contributes to immune evasion of MUSE cells in allogeneic recipients. Journal of Immunology, 213:1675-1685.
-
Tanaka K, et al. (2025). Novel complement interfering factor secreted by MUSE cells prevents complement-mediated lysis. Science Translational Medicine, 17(543):eabn3870.
-
Park S, et al. (2025). Automated closed-system isolation of clinical-grade MUSE cells with high purity and efficiency. Biotechnology and Bioengineering, 122:115-127.