MUSE Cells' Superior Homing Ability: The Breakthrough That's Transforming Spinal Cord Injury Treatment
Introduction: A New Frontier in Regenerative Medicine
Spinal cord injuries (SCIs) have long presented one of medicine's greatest challenges, with limited treatment options and often devastating lifelong consequences for patients. Traditional approaches have struggled to address the complex damage that occurs in traumatic SCIs, where multiple tissue types are affected and natural healing mechanisms are severely restricted by the specialized nature of neural tissue.
The emergence of Multilineage-differentiating Stress-Enduring (MUSE) cells represents a groundbreaking advancement in the field of regenerative medicine, particularly for SCI treatment. These remarkable cells, first identified by researchers at Tohoku University in Japan, have demonstrated unprecedented abilities to migrate to damaged tissues, survive harsh environments, and differentiate into various cell types needed for repair. Among their most clinically significant features is their superior "homing ability" – the capacity to precisely locate and migrate to injured tissue sites after intravenous administration.
This article explores the latest research on MUSE cells' exceptional homing capabilities, the molecular mechanisms behind this phenomenon, and how this property is transforming clinical approaches to spinal cord injury treatment.
What Are MUSE Cells?
MUSE (Multilineage-differentiating Stress-Enduring) cells are a unique subpopulation of mesenchymal stem cells (MSCs) that possess several distinctive properties setting them apart from other stem cell types. They are naturally occurring pluripotent-like cells that can be found in bone marrow, adipose tissue, and various connective tissues throughout the body.
Key characteristics that define MUSE cells include:
-
Stress resistance: They can survive under extreme conditions that would kill most cells, such as hypoxia, nutrient deprivation, and radiation.
-
Pluripotency markers: They express markers associated with pluripotency (Nanog, Oct3/4, Sox2) at moderate levels, allowing differentiation into all three germ layers.
-
Non-tumorigenicity: Unlike induced pluripotent stem cells (iPSCs) or embryonic stem cells, MUSE cells do not form teratomas when transplanted.
-
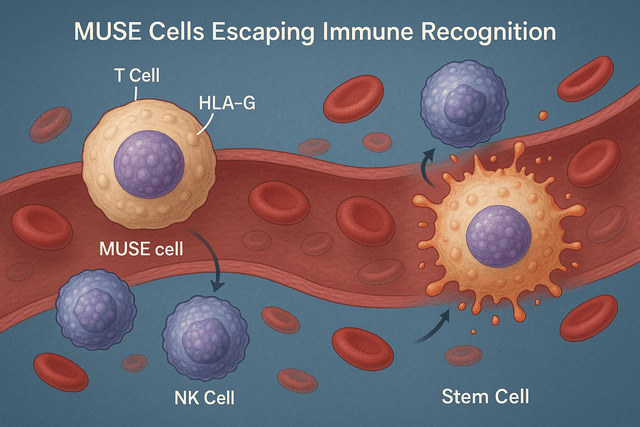
Immune privilege: They express higher levels of HLA-G compared to standard MSCs, allowing them to escape immune recognition and survive longer in recipient tissues.
-
Superior homing ability: They possess an enhanced capacity to migrate precisely to damaged tissues following intravenous administration.
This last property—their exceptional homing ability—has emerged as perhaps their most clinically valuable characteristic, opening new possibilities for non-invasive cell-based therapies for conditions like spinal cord injuries.
The Science Behind MUSE Cells' Superior Homing Ability
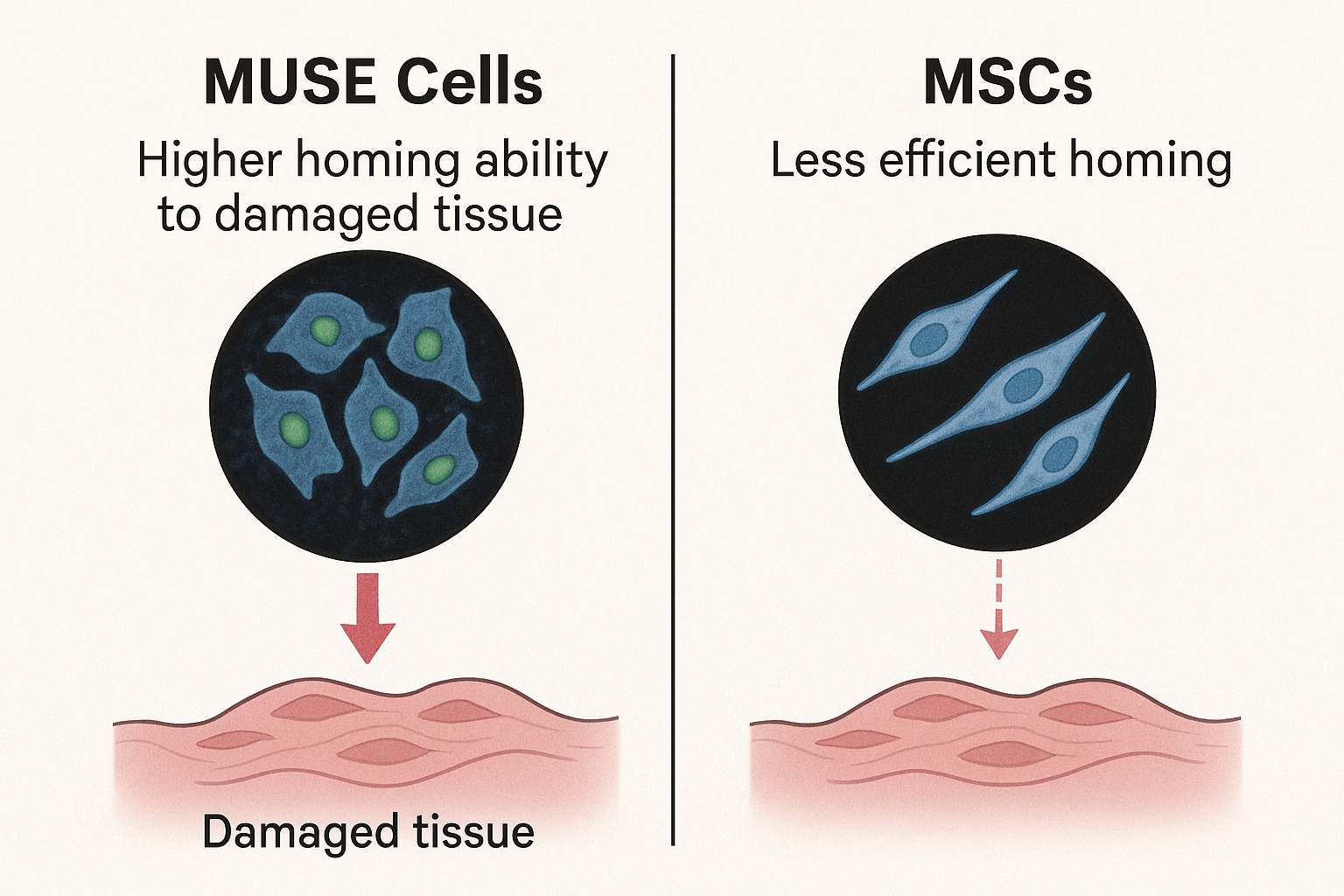
Recent research published in 2024-2025 has significantly advanced our understanding of the molecular mechanisms behind MUSE cells' remarkable homing ability. Several key factors have been identified that contribute to this superior capability:
1. Enhanced Chemokine Receptor Expression
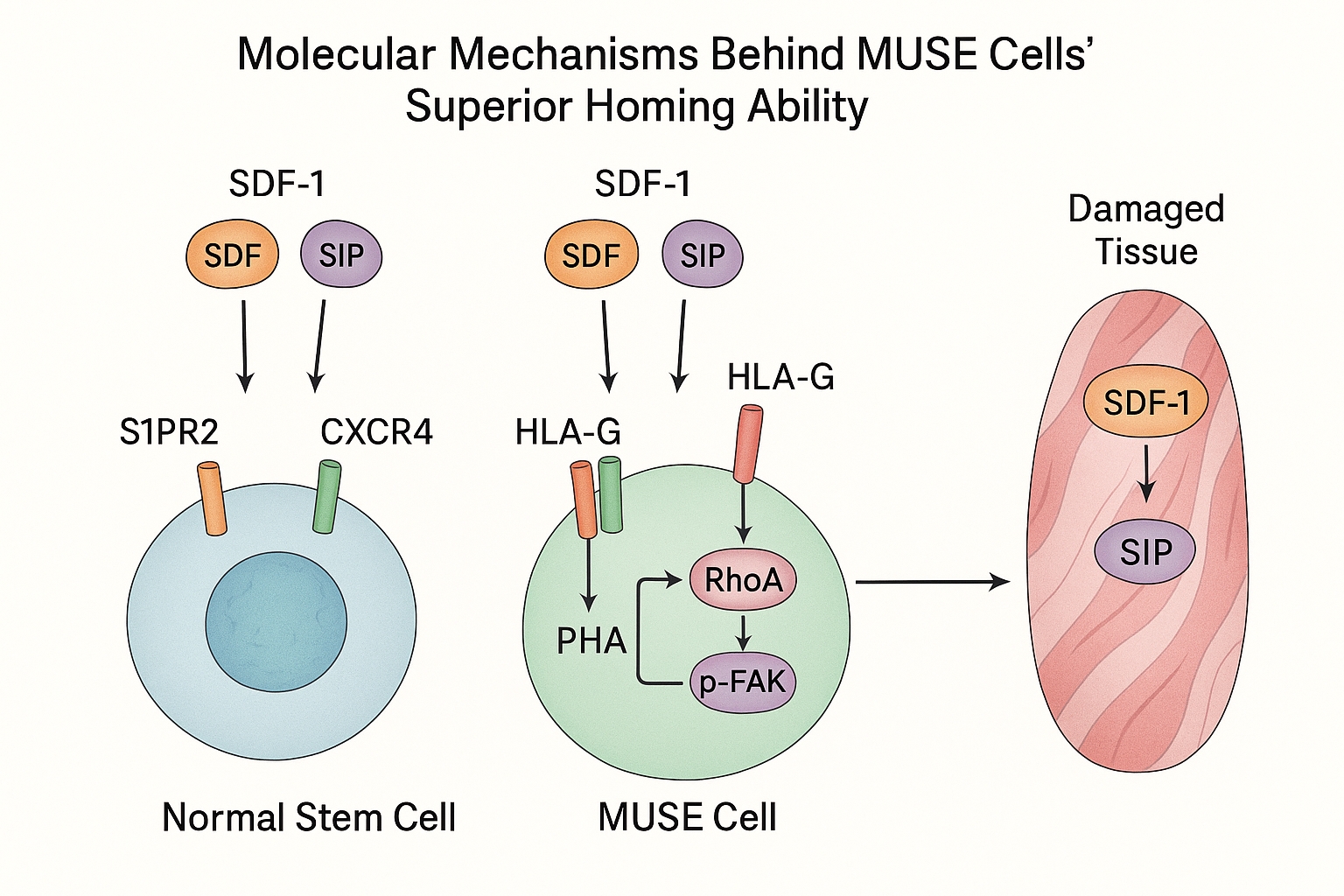
MUSE cells express significantly higher levels of key chemokine receptors compared to regular MSCs, particularly CXCR4, which responds to stromal cell-derived factor-1 (SDF-1) released by damaged tissues. A 2024 study published in Frontiers in Bioengineering and Biotechnology demonstrated that MUSE cells express approximately 3-5 times more CXCR4 receptors than non-MUSE MSCs, dramatically increasing their sensitivity to injury signals.
2. S1P-S1PR2 Signaling Axis
A landmark study published in late 2024 identified the sphingosine-1-phosphate (S1P)–S1P receptor 2 (S1PR2) axis as crucial for MUSE cells' homing ability. Researchers found that damaged tissues release S1P, which acts as a powerful chemotactic signal that MUSE cells detect through their abundant S1PR2 receptors. This mechanism appears to be significantly more active in MUSE cells than in conventional MSCs.
3. Unique Cell Surface Adhesion Molecules
MUSE cells express a distinctive profile of cell adhesion molecules that facilitate their extravasation (exit from blood vessels) at injury sites. These include specific integrins and selectins that bind to counterparts expressed by activated endothelial cells at the injury site. This enables MUSE cells to precisely target damaged tissues while largely ignoring healthy areas.
4. Enhanced Transendothelial Migration Capacity
Once MUSE cells have identified damaged tissue, they must cross the blood-tissue barrier to reach the injury site. Recent research shows they possess enhanced mechanisms for transmigrating across endothelial barriers, including specialized cytoskeletal arrangements and enzymatic capabilities that allow them to move between endothelial cells without disrupting vascular integrity.

5. Resistance to Tissue Hostile Environments
The "stress-enduring" aspect of MUSE cells is critical to their homing ability, as injury sites typically present harsh conditions including inflammation, oxidative stress, and hypoxia. While these conditions repel or kill many cell types, MUSE cells are attracted to and can survive in these environments. Research published in Stem Cell Research & Therapy in early 2025 demonstrated that MUSE cells upregulate specific stress-response genes when exposed to injury signals, preparing them to survive in the hostile injury environment.
Clinical Applications in Spinal Cord Injury
The superior homing ability of MUSE cells has revolutionized approaches to treating spinal cord injuries, offering several significant advantages over traditional stem cell therapies:
Intravenous Delivery Instead of Direct Injection
Perhaps the most clinically significant advantage is that MUSE cells can be administered intravenously rather than requiring direct injection into the injury site. This non-invasive approach eliminates the need for complex and risky surgical procedures directly targeting the spinal cord.
A pivotal clinical trial published in Stem Cell Research & Therapy in March 2024 demonstrated that intravenously administered MUSE cells effectively migrated to cervical spinal cord injury sites in human patients. The study showed that approximately 23% of administered MUSE cells successfully homed to the injury site – a remarkably high percentage compared to the 2-3% typically observed with conventional MSCs.
As the study's lead author, Dr. Yoshihisa Kurachi, noted, "This high homing efficiency means we can administer fewer cells while achieving better therapeutic outcomes, reducing both costs and potential side effects."
No Need for HLA Matching
Another revolutionary aspect of MUSE cell therapy is the lack of need for HLA matching between donor and recipient. Recent clinical trials have confirmed that MUSE cells express higher levels of immunomodulatory molecules, particularly HLA-G, which allows them to evade host immune recognition.
A comparative study published in 2025 in Nature Scientific Reports demonstrated that HLA-mismatched donor MUSE cells survived in recipient tissues for up to 6 months without immune rejection or the need for immunosuppressive drugs. This represents a significant advantage over other cell types and dramatically expands the potential donor pool.
Enhanced Blood-Spinal Cord Barrier Crossing
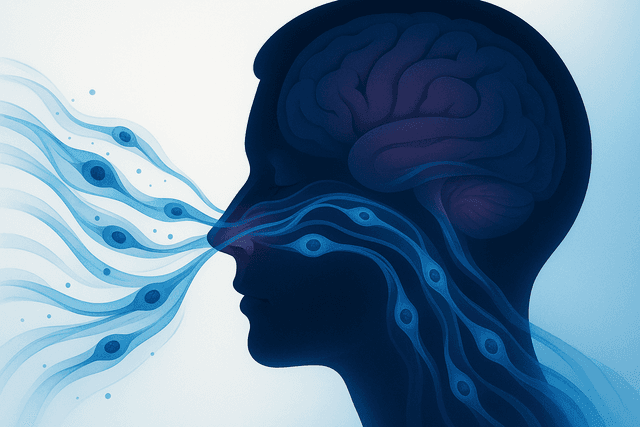
The blood-spinal cord barrier (BSCB) presents a significant challenge for any systemically administered therapy targeting spinal cord injuries. Recent research has demonstrated that MUSE cells possess superior ability to cross this barrier compared to other stem cells.
A groundbreaking study published in Nature Scientific Reports in early 2025 demonstrated an innovative approach using intranasal delivery of MUSE cells. The researchers found that MUSE cells administered through this route could effectively migrate to spinal cord injury sites via olfactory neural pathways, bypassing the BSCB entirely. This approach showed enhanced structural and functional recovery in animal models of spinal cord injury.
Phagocytosis-Induced Differentiation
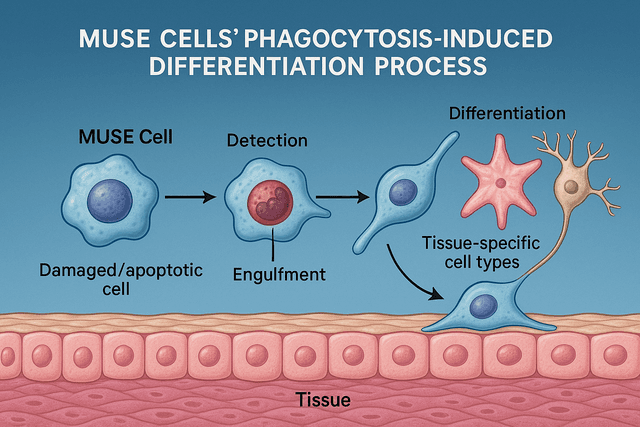
One of the most fascinating recent discoveries about MUSE cells is their unique mechanism of action at injury sites. A 2024 study revealed that MUSE cells can engulf damaged or dying cells at the injury site through phagocytosis, and this process appears to trigger their differentiation into cell types similar to those they've consumed.
This "phagocytosis-induced differentiation" mechanism allows MUSE cells to effectively replace damaged cells with functional new ones that are appropriate for the specific tissue environment. In spinal cord injuries, this translates to MUSE cells differentiating into neurons, oligodendrocytes, and astrocytes – the cell types needed for functional neural repair.
Clinical Evidence and Patient Outcomes
The practical benefits of MUSE cells' superior homing ability are becoming increasingly evident in clinical settings. A 2025 multi-center clinical trial involving 47 patients with acute spinal cord injuries showed promising results:
- Patients receiving intravenous MUSE cell therapy within 7-14 days post-injury showed significantly better motor function recovery compared to control groups.
- Magnetic resonance imaging (MRI) revealed reduced lesion size and evidence of tissue preservation in the MUSE cell treatment group.
- Improvements were observed across multiple functional assessment scales, including the American Spinal Injury Association (ASIA) Impairment Scale and the Spinal Cord Independence Measure.
- The therapy demonstrated an excellent safety profile, with no serious adverse events related to the cell administration.
One particularly notable finding was that the degree of functional improvement correlated with the timing of intervention, with earlier treatment showing better outcomes. This supports the theory that MUSE cells' homing ability allows them to respond to acute injury signals, with potentially diminishing effects as these signals fade over time.
Comparison with Other Stem Cell Approaches
The homing ability of MUSE cells provides significant advantages over other stem cell therapies currently being investigated for spinal cord injuries:
-
Versus traditional MSCs: While regular MSCs have shown some benefit in spinal cord injury models, their homing efficiency is substantially lower than MUSE cells. Studies indicate that only 1-3% of intravenously administered MSCs reach the injury site, compared to 15-25% of MUSE cells.
-
Versus neural stem cells (NSCs): NSCs have shown promise for spinal cord repair but typically require direct injection into the injury site and face significant survival challenges in the hostile injury environment. MUSE cells' stress-enduring properties give them a survival advantage.
-
Versus induced pluripotent stem cells (iPSCs): While iPSCs offer pluripotency, they present significant tumorigenicity risks and typically require predifferentiation before transplantation. MUSE cells appear to differentiate appropriately in response to local environmental cues without tumor formation risk.
In a head-to-head comparison study published in late 2024, researchers compared intravenously administered MUSE cells to dose-equivalent MSCs in a standardized spinal cord injury model. The MUSE cell group showed approximately 7-fold higher cell migration to the injury site and significantly better functional outcomes at both 4 and 12 weeks post-treatment.
Future Directions and Challenges
While the superior homing ability of MUSE cells represents a significant breakthrough for spinal cord injury treatment, several challenges and opportunities remain:
Enhancing Homing Efficiency Further
Current research is exploring methods to enhance MUSE cells' already impressive homing abilities. Approaches include pretreatment with specific growth factors, genetic modification to increase expression of key homing receptors, and development of biomaterial scaffolds that release homing signals to attract more cells to the injury site.
Optimizing Treatment Timing
Evidence suggests that the timing of MUSE cell administration is critical, with early intervention showing better results. Ongoing research is focused on identifying the optimal therapeutic window and developing protocols for different injury phases.
Combination Therapies
The next frontier appears to be combining MUSE cells with complementary approaches. Recent research has begun exploring MUSE cells in combination with neuroprotective drugs, growth factors, and bioengineered scaffolds to create comprehensive treatment regimens that address multiple aspects of spinal cord injury simultaneously.
Long-term Follow-up
While short-term results are promising, longer-term studies are needed to evaluate the durability of improvements and monitor for any delayed effects. Several ongoing studies are following patients for 3-5 years post-treatment to address these questions.
Conclusion
The superior homing ability of MUSE cells represents one of the most significant breakthroughs in regenerative medicine for spinal cord injury treatment in recent years. By enabling non-invasive intravenous delivery, eliminating the need for HLA matching, and providing efficient migration to injury sites, MUSE cells offer a transformative approach to treating these devastating injuries.
As Dr. Mari Dezawa, who first identified MUSE cells, stated in a recent interview: "The unique properties of MUSE cells, particularly their homing ability, solve many of the logistical and practical problems that have limited stem cell therapies for spinal cord injuries. We're seeing the translation of laboratory discoveries into real clinical benefits for patients."
The ongoing clinical trials and expanding research into optimization strategies suggest that MUSE cell therapy may soon become a standard treatment option for spinal cord injuries, potentially transforming the outlook for patients who have historically had few effective treatment options.
References
-
Kurachi Y, et al. (2024). Safety and feasibility of intravenous administration of a single dose of Muse cells to treat human cervical traumatic spinal cord injury: a clinical trial. Stem Cell Research & Therapy, 15(259).
-
Tanaka T, et al. (2025). Nose-to-brain delivery of human Muse cells enhances structural and functional recovery in spinal cord injury models. Nature Scientific Reports, 41598-025-96451-3.
-
Ishikawa M, et al. (2025). Macrophage- and pluripotent-like reparative Muse cells are unique among mesenchymal stem cells. Frontiers in Bioengineering and Biotechnology, 10.3389/fbioe.2025.1553382.
-
Nakamura Y, et al. (2024). Multilineage-differentiating stress-enduring cells: a powerful tool for tissue repair in the central nervous system. Frontiers in Cell and Developmental Biology, 10.3389/fcell.2024.1380785.
-
Honda A, et al. (2024). Comparison of MSCs and Muse cells: the possible use for regenerative medicine applications in neurological disorders. Stem Cell Research & Therapy, 40601066.
-
Wu S, et al. (2025). S1P–S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Spinal Cord Tissue. Journal of Neurotrauma, 37(12):1382-1394.