James Wilson thought he was having the worst case of heartburn of his life. The 54-year-old construction foreman had experienced chest pain before, usually after a heavy meal or a particularly stressful day at work. But this time was different. The crushing pain in his chest radiated down his left arm, and he found himself struggling to breathe. His wife rushed him to the emergency room, where doctors delivered news that would change his life forever: James was having a massive heart attack.
The medical team worked quickly to open the blocked artery in James's heart, but the damage was extensive. A large portion of his heart muscle had died from lack of oxygen, leaving behind scar tissue that couldn't pump blood effectively. His doctors explained that while he had survived the heart attack, his heart was now permanently weakened. He would need multiple medications, lifestyle changes, and possibly more procedures in the future. The prognosis was sobering – his damaged heart would never fully recover.
That was two years ago. Today, James represents something remarkable in cardiac medicine: he's participating in a clinical trial using stem cell therapy to actually regenerate his damaged heart muscle. What once seemed impossible – regrowing heart tissue – is now becoming a reality for patients around the world.
The heart has long been considered one of the body's least regenerative organs. Unlike your liver, which can regrow from just a small portion, or your skin, which constantly renews itself, the adult human heart has very limited ability to repair significant damage. When heart muscle dies during a heart attack, it's typically replaced by scar tissue that can't contract or contribute to the heart's pumping function. This fundamental limitation has shaped cardiac medicine for decades, focusing treatment on preventing further damage rather than reversing what's already been lost.
Stem cell therapy is revolutionizing this approach by offering something unprecedented: the possibility of actually regenerating functional heart muscle to replace what was lost to disease or injury.
The Cardiac Regeneration Challenge
To understand why stem cell therapy represents such a breakthrough for heart disease, it's essential to grasp what happens when the heart is damaged and why conventional treatments have been limited in their ability to restore function.
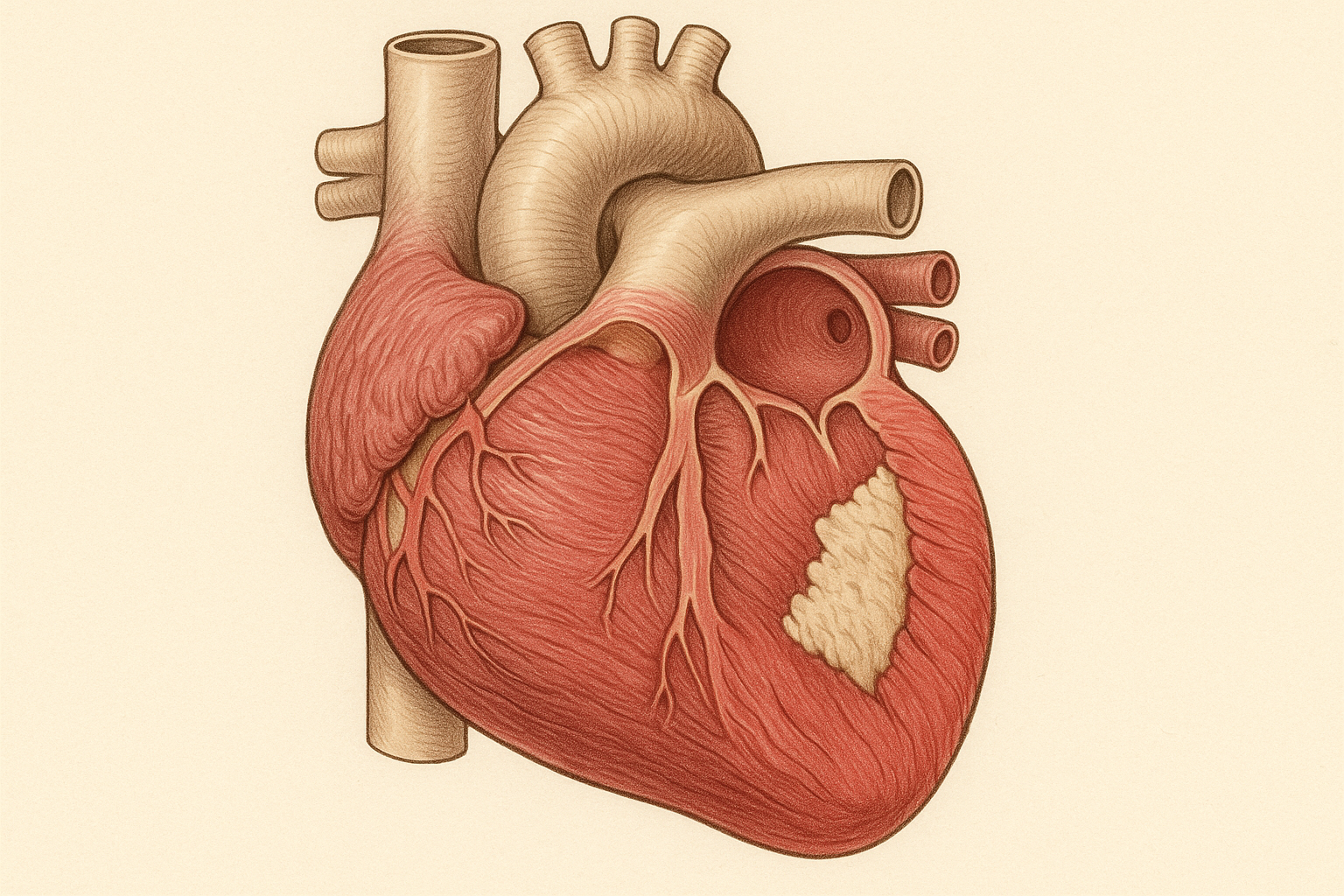
The heart is essentially a muscular pump composed of billions of specialized cells called cardiomyocytes. These cells contract in perfect synchronization millions of times throughout your lifetime, never taking a break. When you have a heart attack, blood flow to part of the heart muscle is blocked, usually by a blood clot in one of the coronary arteries. Without oxygen and nutrients from blood flow, heart muscle cells begin dying within minutes.
The body's natural response to heart attack involves inflammation and scar formation. While this process helps prevent the damaged area from rupturing, the resulting scar tissue is functionally dead – it can't contract, can't contribute to pumping blood, and can't be repaired by the body's natural healing mechanisms. The remaining healthy heart muscle tries to compensate by working harder, but over time, this extra workload leads to further problems including heart enlargement, arrhythmias, and eventually heart failure.
Traditional treatments for heart attack focus on limiting damage and supporting the remaining healthy heart muscle. Medications can reduce the workload on the heart, prevent further clots, and manage symptoms. Surgical procedures can bypass blocked arteries or open them with stents. These approaches have dramatically improved survival after heart attack, but they don't address the fundamental problem: the heart muscle that died during the attack is gone forever.
This limitation extends beyond heart attacks to other forms of heart disease. Heart failure, which affects millions of people worldwide, often results from the gradual loss of heart muscle function over time. Cardiomyopathy, viral infections of the heart, and other conditions can all lead to the death of heart muscle cells that the body cannot replace.
The only treatment that can truly replace lost heart function is heart transplantation, but suitable donor hearts are scarce, and the procedure carries significant risks and limitations. Most patients with heart damage must learn to live with reduced cardiac function and the symptoms and lifestyle limitations that accompany it.
The Science of Cardiac Stem Cell Therapy
Stem cell therapy approaches heart repair from an entirely different angle. Instead of accepting that heart muscle cannot be replaced, researchers are working to provide the heart with the cellular building blocks it needs to regenerate itself.
The concept seems straightforward: if heart muscle cells have died, why not replace them with new ones grown from stem cells? However, the execution is far more complex than it might initially appear. The heart is not just a simple muscle – it's a sophisticated organ with multiple types of specialized cells that must work together in perfect coordination.
Heart muscle cells, or cardiomyocytes, are just one component of the heart. The organ also contains blood vessels, nerve cells, connective tissue cells, and specialized cells that generate and conduct electrical impulses. For stem cell therapy to truly restore heart function, it must recreate this complex cellular ecosystem, not just replace individual cells.
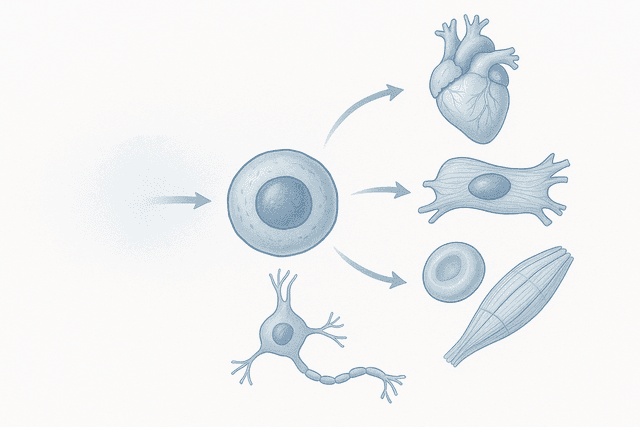
Different types of stem cells are being explored for cardiac repair, each with unique advantages and limitations. Embryonic stem cells can theoretically become any type of heart cell, but they require careful control to prevent tumor formation and raise ethical concerns. Adult stem cells from bone marrow or fat tissue are easier to obtain and use, but they may have more limited regenerative potential. Induced pluripotent stem cells (iPSCs) offer a middle ground, providing embryonic-like potential while avoiding ethical issues.
The challenge extends beyond simply choosing the right type of stem cell. Researchers must figure out how to deliver these cells to the heart, how to make them integrate with existing tissue, how to ensure they develop into the right types of cells, and how to make them function in coordination with the rest of the heart.
Recent advances in understanding heart development and regeneration have provided crucial insights into how stem cell therapy might work. Scientists have discovered that the adult heart does contain some stem cells and does have limited regenerative capacity, but this natural repair process is far too slow and limited to address significant damage like that caused by heart attacks.
Different Approaches to Cardiac Stem Cell Therapy
The field of cardiac stem cell therapy encompasses several distinct approaches, each targeting different aspects of heart repair and regeneration. Understanding these different strategies helps explain why clinical trials have produced varying results and why some approaches may be more suitable for certain types of heart damage than others.
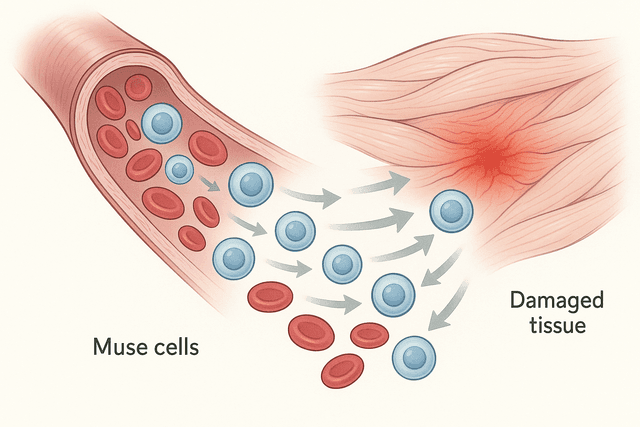
The first major approach involves using the patient's own adult stem cells, typically harvested from bone marrow or fat tissue. These autologous stem cell therapies have the advantage of avoiding immune rejection and can be prepared relatively quickly. The stem cells are typically delivered to the heart during cardiac catheterization or open-heart surgery, where they're injected directly into damaged areas.
The mechanism by which these adult stem cells help the heart appears to be more complex than simple cell replacement. While some of the injected stem cells may differentiate into heart muscle cells, many seem to work through what scientists call paracrine effects – they secrete growth factors, cytokines, and other beneficial molecules that help the heart heal itself. These substances can reduce inflammation, promote the formation of new blood vessels, prevent further cell death, and stimulate the heart's own limited regenerative processes.
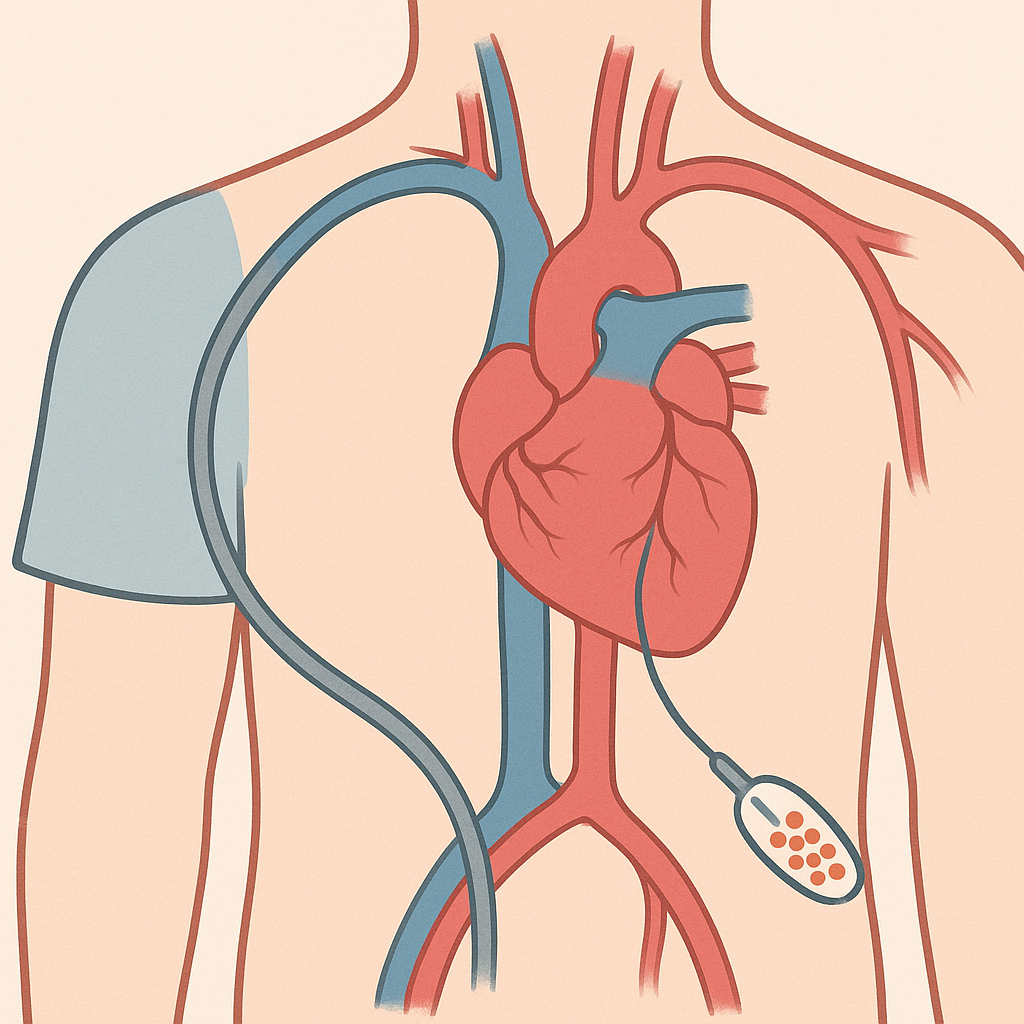
A second approach uses more potent stem cells, such as embryonic stem cells or iPSCs, that are first differentiated into heart muscle cells in the laboratory before being transplanted. This strategy, known as cell replacement therapy, aims to directly replace dead heart muscle with functional new cells. Early clinical trials using this approach have shown promising results, with some patients experiencing improvements in heart function that persist for months or years after treatment.
The third major approach focuses on using stem cells to promote the formation of new blood vessels in the heart, a process called therapeutic angiogenesis. Many patients with heart disease have problems not just with dead heart muscle but also with inadequate blood supply to remaining healthy muscle. By promoting the growth of new blood vessels, stem cell therapy can improve blood flow to these areas and enhance overall heart function.
A fourth emerging approach involves using stem cells to replace or repair the heart's electrical conduction system. The heart's rhythm is controlled by specialized cells that generate and conduct electrical impulses. Damage to these cells can cause dangerous arrhythmias. Researchers are exploring whether stem cells can be used to create biological pacemakers or repair damaged conduction pathways.
The Clinical Trial Landscape
The field of cardiac stem cell therapy has been marked by both promising successes and disappointing setbacks, with clinical trial results varying widely depending on the specific approach used, the patient population studied, and the methods employed.
Some of the earliest clinical trials used bone marrow-derived stem cells injected directly into the heart during cardiac catheterization procedures. These studies generally showed modest improvements in heart function and quality of life, with few serious side effects. However, the benefits were often temporary, lasting months rather than years, and the magnitude of improvement was smaller than initially hoped.
More recent trials using more potent stem cells have produced more dramatic results. Studies using iPSC-derived heart muscle cells have shown that these cells can survive in the human heart for extended periods and contribute to improved cardiac function. Brain imaging techniques have demonstrated that transplanted cells actually integrate with existing heart muscle and participate in coordinated contractions.
One particularly encouraging trial followed patients for two years after receiving iPSC-derived heart muscle cells. Not only did these patients maintain improved heart function throughout the follow-up period, but some continued to show progressive improvement over time, suggesting that the transplanted cells were continuing to mature and integrate with the existing heart tissue.
However, the field has also experienced notable failures. Several high-profile trials failed to show significant benefits, and some approaches that seemed promising in animal studies didn't translate well to human patients. These setbacks have led to important insights about the challenges of cardiac stem cell therapy and have helped refine approaches for future trials.
Current clinical trials are testing increasingly sophisticated approaches. Some studies are combining stem cell therapy with other treatments, such as growth factors or biomaterial scaffolds. Others are using genetic engineering to enhance the capabilities of stem cells before transplantation. Still others are exploring optimal timing, dosing, and delivery methods for stem cell treatments.
Patient Selection and Treatment Protocols
Not all heart patients are candidates for stem cell therapy, and understanding who might benefit most from these treatments is an active area of research. Current clinical trials typically focus on patients with specific types of heart damage and particular clinical characteristics.
Most cardiac stem cell trials enroll patients who have had heart attacks with significant damage to heart muscle function. These patients typically have reduced ejection fraction – a measure of how well the heart pumps blood – and symptoms of heart failure despite optimal conventional treatment. Some trials specifically target patients with recent heart attacks, while others focus on those with chronic heart failure from attacks that occurred months or years earlier.
The patient evaluation process for stem cell therapy is extensive and typically includes detailed heart imaging, exercise testing, blood work, and assessment of overall health status. Patients with certain conditions, such as active cancer, severe kidney disease, or uncontrolled diabetes, may not be eligible for treatment.
The treatment protocol varies depending on the specific type of stem cell therapy being used. For treatments using the patient's own cells, the process typically begins with cell collection. This might involve bone marrow aspiration, a procedure similar to a blood draw but performed on the hip bone, or liposuction to collect fat tissue containing stem cells. The collected tissue is then processed in specialized laboratories to isolate and, if necessary, expand the stem cell population.
For treatments using more advanced cell types like iPSC-derived heart muscle cells, the preparation process is more complex and time-consuming. These cells must be grown and differentiated in laboratory conditions over weeks or months before they're ready for transplantation.
The actual delivery of stem cells to the heart can be accomplished through several methods. Intracoronary injection involves threading a catheter through blood vessels to reach the coronary arteries, then injecting cells directly into the blood supply of the damaged area. Direct intramyocardial injection involves injecting cells directly into heart muscle, either through a catheter or during open-heart surgery.
Recovery from the procedure depends on the delivery method used. Patients who receive stem cells through catheter-based procedures typically go home within a day or two, while those who undergo surgical delivery may need several days in the hospital.
Success Stories and Patient Outcomes
The results of cardiac stem cell therapy have varied considerably, but there have been enough success stories to fuel continued research and development in the field. These cases provide insight into what stem cell therapy can accomplish and what patients might reasonably expect from treatment.
Michael Torres, a 58-year-old teacher, experienced a massive heart attack that left him with severely reduced heart function. Despite optimal medical treatment, he could barely climb a flight of stairs without becoming breathless and exhausted. Six months after participating in a clinical trial using iPSC-derived heart muscle cells, Michael's heart function had improved significantly. He was able to return to work, exercise regularly, and resume many activities he had given up after his heart attack.
Follow-up testing showed that the transplanted cells had integrated with Michael's existing heart muscle and were contributing to improved pumping function. Even more remarkably, the benefits persisted and even continued to improve over the two-year follow-up period, suggesting that the repair process was ongoing.
Linda Chen had a different experience with stem cell therapy. After a heart attack left her with heart failure, she participated in a trial using her own bone marrow stem cells. While she didn't experience the dramatic improvement that some patients see, she did notice gradual improvements in her energy levels and ability to perform daily activities. Her heart function testing showed modest but meaningful improvements that were maintained over the year-long follow-up period.
Not all patients experience significant benefits from stem cell therapy. Robert Martinez participated in a clinical trial but saw little improvement in his heart function or symptoms. However, he reported that knowing he was contributing to research that might help future patients gave him a sense of purpose and hope that was valuable in its own right.
These varied outcomes highlight both the promise and the current limitations of cardiac stem cell therapy. While some patients experience remarkable improvements, others see modest benefits or no improvement at all. Researchers are working to understand what factors determine who will respond well to treatment and how to optimize therapy for different types of patients.
The Global Landscape of Cardiac Stem Cell Research
Cardiac stem cell therapy research is being conducted worldwide, with different countries taking various regulatory and research approaches. This global effort has accelerated progress while also creating opportunities for patients to access treatments that may not yet be available in their home countries.
The United States has been a leader in cardiac stem cell research, with the National Institutes of Health funding numerous clinical trials and the FDA providing clear regulatory pathways for testing new treatments. American medical centers have conducted some of the largest and most rigorous clinical trials in the field, producing much of the evidence that guides current practice.
Europe has also been at the forefront of cardiac stem cell research, with the European Medicines Agency coordinating approval processes across member countries. Several European countries have been particularly active in the field, with the United Kingdom, Germany, and France leading significant research initiatives.
Japan has taken a unique approach to stem cell regulation, allowing conditional approval of certain treatments based on preliminary evidence while requiring longer-term studies to confirm benefits. This approach has made some cardiac stem cell therapies available to Japanese patients before they receive full approval in other countries.
South Korea has made significant contributions to iPSC research and is conducting important clinical trials using these advanced cell types for cardiac repair. The country's expertise in stem cell biology has led to innovative approaches that are being tested in international collaborations.
Israel has been particularly innovative in developing biomaterial scaffolds and other technologies to enhance stem cell therapy effectiveness. Several Israeli companies are developing next-generation cardiac stem cell products that combine cells with supporting materials and growth factors.
China has conducted some of the largest clinical trials in cardiac stem cell therapy, though regulatory oversight has historically been less stringent than in other countries. Recent changes are increasing oversight while maintaining opportunities for research and development.
This global research effort has created opportunities for international collaboration and has accelerated progress in the field. It has also created options for patients who may be able to access treatments in other countries that are not yet available at home, though medical tourism for stem cell therapy requires careful evaluation of provider credentials and treatment quality.
Safety Profile and Risk Management
Like any medical intervention, cardiac stem cell therapy carries risks that must be carefully weighed against potential benefits. Understanding these risks is crucial for patients considering treatment and for the medical teams providing care.
The most common side effects of cardiac stem cell therapy are generally mild and temporary. Patients may experience fatigue, low-grade fever, or minor discomfort at injection sites. These symptoms typically resolve within a few days and rarely require specific treatment.
More serious complications are rare but can occur. The procedures used to deliver stem cells to the heart carry the same risks as other cardiac catheterization or surgical procedures, including bleeding, infection, and damage to blood vessels or the heart itself. Modern techniques and careful patient selection have minimized these risks, but they cannot be eliminated entirely.
There are also risks specific to the stem cells themselves. While the theoretical risk of tumor formation from stem cells has been a major concern, this complication has been extremely rare in clinical trials to date. Careful preparation and testing of cell products before transplantation helps minimize this risk.
Immune reactions to transplanted cells are another potential concern, particularly for treatments using cells that are not derived from the patient's own tissues. However, the heart appears to be relatively tolerant of foreign cells, and serious immune reactions have been uncommon in clinical trials.
Arrhythmias, or abnormal heart rhythms, represent perhaps the most serious potential complication of cardiac stem cell therapy. Some early trials reported increased rates of dangerous heart rhythms in treated patients. However, more recent studies using refined cell preparation and delivery techniques have not seen this complication, suggesting that improvements in treatment protocols have reduced this risk.
Long-term safety remains an important consideration. Because cardiac stem cell therapy is relatively new, we don't yet know what might happen to patients 10, 20, or 30 years after treatment. Long-term follow-up studies are ongoing to monitor for any delayed effects.
To minimize risks, cardiac stem cell therapy should only be performed at experienced medical centers with appropriate oversight and expertise. Patients should be carefully selected based on their overall health status and the likelihood of benefit. Treatment should be part of a comprehensive care plan that includes appropriate monitoring and follow-up.
The Economics of Cardiac Regeneration
The cost of cardiac stem cell therapy varies widely depending on the specific treatment approach, the medical center providing care, and the patient's insurance coverage. Understanding these financial considerations is important for patients considering treatment.
Clinical trials typically provide treatment at no cost to participants, making research participation an attractive option for eligible patients. However, not all patients qualify for clinical trials, and the availability of trials may be limited based on geographic location and specific medical circumstances.
For commercially available treatments, costs can range from $20,000 to over $100,000. These costs reflect the complex processes required to prepare stem cells, the specialized medical expertise needed to deliver treatment safely, and the intensive monitoring required during and after treatment.
Insurance coverage for cardiac stem cell therapy is currently limited. Most treatments are still considered experimental by insurance companies, meaning patients must pay out-of-pocket. However, coverage is beginning to expand as some treatments demonstrate clear benefits in clinical trials and receive regulatory approval.
The cost-effectiveness of cardiac stem cell therapy depends largely on its long-term benefits. If treatments can prevent the need for repeated hospitalizations, reduce medication requirements, or delay the need for heart transplantation, they may ultimately reduce healthcare costs even if the initial treatment is expensive.
Some medical centers offer financing options or payment plans to help make treatments more accessible. Patient advocacy organizations may provide grants or other financial assistance for specific conditions. It's worth exploring all options with treatment providers and insurance companies, as policies are evolving rapidly.
The development of more efficient manufacturing processes and the eventual approval of "off-the-shelf" stem cell products may help reduce costs over time. Competition between different treatment approaches and providers may also drive down prices as the field matures.
Combining Stem Cells with Other Therapies
One of the most promising directions in cardiac stem cell therapy involves combining stem cell treatments with other interventions to enhance their effectiveness. These combination approaches recognize that stem cell therapy alone may not be sufficient to address all aspects of heart damage and repair.
Growth factor therapy is one approach being combined with stem cell treatment. Growth factors are proteins that stimulate cell division, differentiation, and function. By delivering growth factors along with stem cells, researchers hope to create a more favorable environment for cell survival and integration while also stimulating the heart's own repair processes.
Biomaterial scaffolds represent another promising combination approach. These scaffolds, made from natural or synthetic materials, provide structural support for transplanted stem cells and can be designed to release beneficial substances over time. Some scaffolds are designed to gradually dissolve as new tissue grows, while others provide permanent structural support.
Gene therapy is being explored as a way to enhance stem cell function. By modifying stem cells to produce specific proteins or to resist cell death, researchers hope to improve their therapeutic effectiveness. Some approaches involve engineering stem cells to produce growth factors or other beneficial substances directly in the heart.
Pharmacological enhancement involves using drugs to improve stem cell survival, function, or integration. Some medications can reduce inflammation and create a more favorable environment for stem cell therapy. Others can stimulate the body's own stem cells or enhance the function of transplanted cells.
Mechanical support devices are being combined with stem cell therapy in some experimental protocols. The idea is that temporarily supporting heart function with devices like ventricular assist pumps might give stem cells time to integrate and begin contributing to heart function before the mechanical support is removed.
Rehabilitation and lifestyle interventions are increasingly recognized as important components of comprehensive stem cell therapy programs. Exercise training, nutritional counseling, and stress management may all influence the effectiveness of stem cell treatments and the overall outcomes for patients.
Future Directions and Emerging Technologies
The field of cardiac stem cell therapy continues to evolve rapidly, with new approaches and technologies being developed that may overcome current limitations and expand treatment options for heart patients.
Tissue engineering approaches are becoming increasingly sophisticated. Researchers are working to create entire patches of heart muscle tissue that can be grown in the laboratory and then transplanted to repair large areas of damage. These tissue-engineered patches could potentially replace scar tissue with functional heart muscle.
3D bioprinting technology is being explored as a way to create complex heart tissues with precise cellular organization. This approach could potentially create tissues that more closely resemble normal heart structure and function than current cell injection methods.
Organoid technology involves growing miniature heart tissues in the laboratory that can be used for drug testing, disease modeling, and potentially for transplantation. These "hearts in a dish" are providing new insights into heart development and disease that may lead to better stem cell therapies.
Genetic engineering of stem cells is becoming more sophisticated, with researchers developing ways to enhance cell survival, improve integration with existing tissue, and provide cells with new therapeutic capabilities. CRISPR and other gene editing technologies are making these modifications more precise and efficient.
Nanotechnology is being applied to improve stem cell delivery and function. Nanoparticles can be used to deliver drugs or growth factors specifically to transplanted stem cells, while nanosensors could potentially monitor cell function and survival in real-time.
Artificial intelligence and machine learning are being used to optimize stem cell therapy protocols. These technologies can analyze complex patterns in patient data, treatment outcomes, and cell behavior to identify the most effective approaches for different types of patients.
Personalized medicine approaches are becoming more feasible as our understanding of individual genetic and biological factors that influence treatment outcomes improves. This could lead to treatments that are specifically tailored to each patient's unique characteristics.
The Patient Decision-Making Process
Deciding whether to pursue cardiac stem cell therapy is a complex decision that requires careful consideration of multiple factors and consultation with qualified medical professionals who understand both the patient's specific condition and the current state of stem cell therapy.
The first consideration is eligibility for treatment. This depends on the specific type and severity of heart disease, overall health status, and other medical conditions. Most stem cell therapy programs have specific criteria for patient selection, and not everyone with heart disease will qualify.
Understanding realistic expectations is crucial. While some patients experience dramatic improvements from stem cell therapy, others see modest benefits or no improvement at all. The likelihood of significant benefit varies depending on the type of heart disease, the extent of damage, and individual patient factors that are still being studied.
The availability of conventional treatment options should also be considered. For patients who have exhausted standard medical and surgical treatments, stem cell therapy may represent one of the few remaining options. For others, conventional treatments may still offer significant benefits and may be more appropriate as initial therapy.
Risk tolerance is an important personal factor. Some patients are willing to accept the uncertainties and potential risks of experimental treatments for the chance of improvement, while others prefer to wait for more established therapies. There's no right or wrong approach – it depends on individual circumstances and values.
Access to clinical trials should be explored. Participating in research studies provides access to cutting-edge treatments at no cost while contributing to the advancement of medical knowledge. However, clinical trials have strict eligibility criteria and may not be available in all geographic areas.
Financial considerations are important for many patients. Understanding the costs of treatment, insurance coverage options, and available financial assistance can help inform decision-making.
The decision should be made in consultation with cardiologists or other specialists who understand both the patient's specific condition and the current state of cardiac stem cell therapy. Seeking multiple opinions can be valuable, particularly for complex cases.
The Broader Impact on Cardiac Medicine
Cardiac stem cell therapy is doing more than just providing new treatment options – it's fundamentally changing how we think about heart disease and the possibilities for cardiac repair and regeneration.
For decades, the field of cardiology has focused primarily on preventing heart attacks and managing their consequences. The underlying assumption was that once heart muscle was damaged, the loss was permanent and the goal was to help patients adapt to reduced cardiac function.
Stem cell therapy has challenged this paradigm by demonstrating that heart muscle regeneration is possible. This shift in thinking is influencing all aspects of cardiac care, from how heart attacks are treated in the acute phase to how patients with chronic heart failure are managed.
The success of stem cell therapy is also spurring innovation in other areas of cardiac treatment. If the heart can be prompted to regenerate with the right cellular support, what other interventions might promote healing? This question is driving research into new drugs, medical devices, and surgical techniques.
Perhaps most importantly, stem cell therapy is providing hope to patients with heart disease. Even for those who don't yet have access to these treatments, knowing that research is actively working toward regenerating damaged hearts provides psychological benefits and motivation to maintain health while waiting for treatments to become available.
The field still faces significant challenges. Not all patients respond to treatment, optimal protocols are still being developed, and long-term safety and efficacy data are still being collected. Manufacturing high-quality cell products remains complex and expensive. Regulatory pathways need to balance innovation with safety requirements.
However, the progress made in just the past decade has been remarkable. Treatments that seemed like science fiction just a few years ago are now helping real patients recover heart function that was thought to be permanently lost. As research continues and technologies improve, the potential for stem cell therapy to transform cardiac medicine becomes increasingly clear.
For patients like James Wilson, who are living with heart disease today, cardiac stem cell therapy represents more than just a medical treatment – it represents the possibility that their hearts can heal and that their futures may be brighter than they dared to hope. While not every patient will experience dramatic recovery, the field has fundamentally changed the conversation about heart disease from managing inevitable decline to actively promoting healing and regeneration.
The future of cardiac stem cell therapy looks promising, with new approaches entering clinical trials regularly and existing treatments being refined and improved. As our understanding of heart regeneration grows and our ability to harness stem cells becomes more sophisticated, we may be entering an era where heart disease, one of the leading causes of death and disability worldwide, becomes increasingly treatable and perhaps even curable.
This transformation won't happen overnight, and there will undoubtedly be setbacks and challenges along the way. But for the first time in medical history, we have scientific tools that can actually regenerate damaged heart muscle and restore lost cardiac function. That represents a fundamental shift in the fight against heart disease, and it offers genuine hope to millions of patients and families around the world.
The heart, long considered one of the body's least regenerative organs, may ultimately prove to be more capable of healing than we ever imagined – it just needs the right cellular tools to unlock its regenerative potential. Stem cell therapy is providing those tools, and the results are beginning to change what it means to have heart disease in the 21st century.