Introduction: A Watershed Moment for Regenerative Medicine
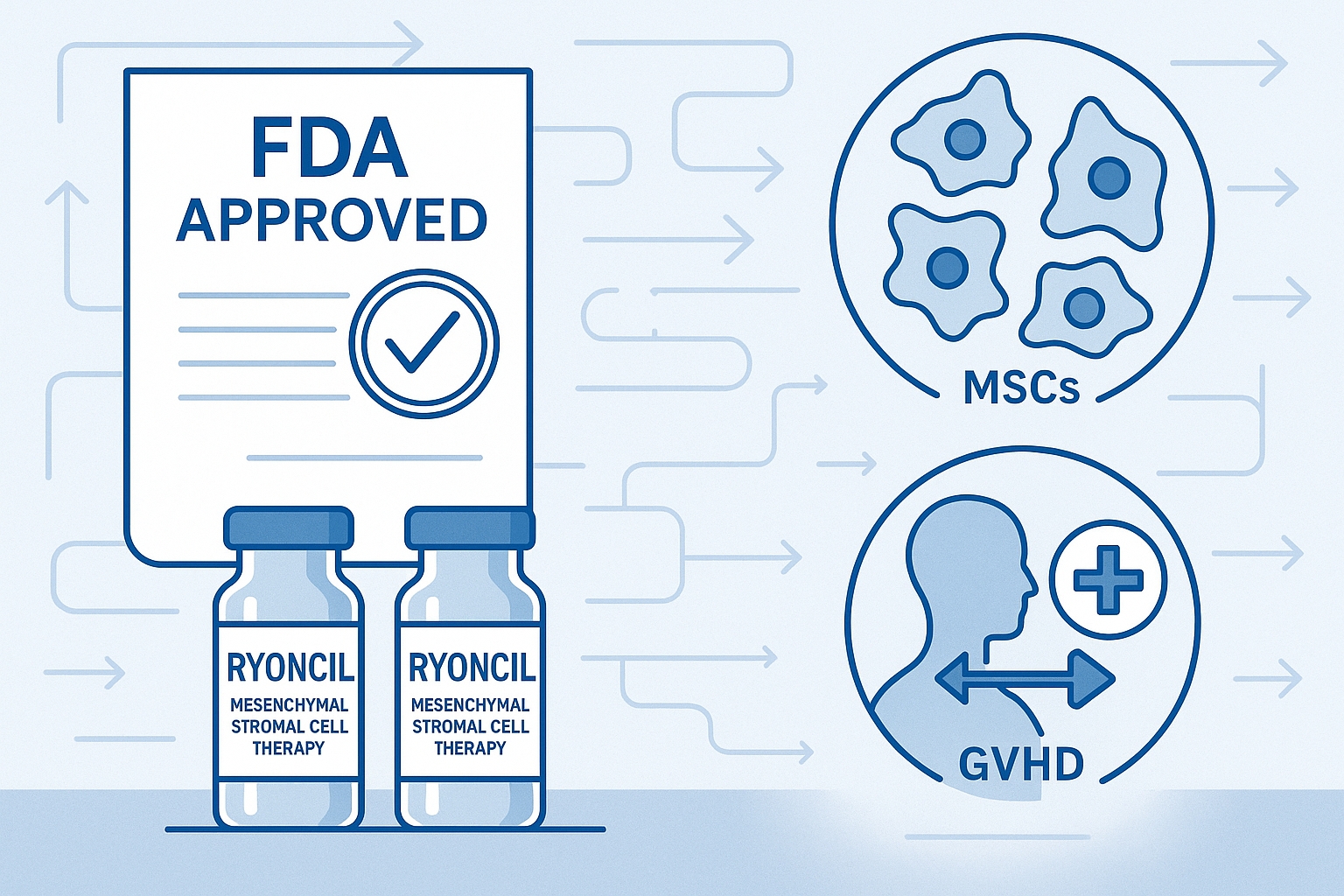
In December 2024, the U.S. Food and Drug Administration (FDA) made a historic decision that marked a turning point for regenerative medicine: the approval of Ryoncil (remestemcel-L), the first mesenchymal stromal cell (MSC) therapy to receive full approval in the United States. This groundbreaking therapy, developed by Mesoblast Limited, is indicated for the treatment of steroid-refractory acute graft-versus-host disease (SR-aGvHD) in pediatric patients 2 months of age and older.
While cell therapies like CAR-T have previously gained FDA approval for cancer treatment, Ryoncil represents the first approval of an off-the-shelf allogeneic MSC product—a development with profound implications that extend far beyond its immediate indication. This regulatory milestone not only offers new hope for vulnerable pediatric patients with a life-threatening condition but also establishes crucial regulatory precedents that could accelerate the development of numerous MSC and other cell-based therapies in the pipeline.
This article explores the significance of Ryoncil's approval, the science behind MSC therapy for graft-versus-host disease, the regulatory pathway that led to this milestone, and the broader implications for the future of regenerative medicine.
Understanding Ryoncil and Mesenchymal Stromal Cells
What Are Mesenchymal Stromal Cells?
Mesenchymal stromal cells (MSCs) are multipotent adult stem cells found in various tissues, including bone marrow, adipose tissue, umbilical cord, and placenta. They possess several remarkable properties that make them valuable for therapeutic applications:
-
Immunomodulation: MSCs can regulate immune responses by interacting with various immune cells, including T cells, B cells, natural killer cells, and dendritic cells.
-
Tissue repair: They secrete growth factors, cytokines, and extracellular vesicles that promote tissue repair and regeneration.
-
Homing to damaged tissue: When administered systemically, MSCs can migrate to sites of injury or inflammation.
-
Low immunogenicity: MSCs express low levels of MHC class I and minimal MHC class II and costimulatory molecules, allowing them to evade immune recognition.
-
Expansibility: They can be expanded in laboratory settings to generate therapeutic doses from a single donor.
These properties have made MSCs one of the most extensively studied cell types in regenerative medicine, with over 1,000 clinical trials exploring their potential across numerous conditions.
Ryoncil: From Laboratory to Approved Therapy
Ryoncil (remestemcel-L) consists of culture-expanded MSCs derived from the bone marrow of healthy adult donors. The manufacturing process involves isolating MSCs from donor bone marrow, expanding them in controlled laboratory conditions, and cryopreserving them for off-the-shelf use without the need for HLA matching.
What sets Ryoncil apart from experimental MSC products is its standardized manufacturing process, which ensures consistent cell quality, potency, and safety. Each dose contains approximately 2 million MSCs per kilogram of patient weight, administered intravenously.
A comprehensive characterization study published in Cytotherapy in early 2024 detailed Ryoncil's unique properties:
- Enhanced expression of immunomodulatory proteins compared to standard MSCs
- Consistent secretion profiles of key anti-inflammatory cytokines
- Standardized cell surface marker expression meeting International Society for Cell & Gene Therapy criteria
- Verified potency in functional T-cell suppression assays
These characteristics contribute to Ryoncil's efficacy in managing the runaway inflammation seen in steroid-refractory acute GvHD.
Graft-Versus-Host Disease: A Life-Threatening Complication
To appreciate the significance of Ryoncil's approval, it's essential to understand the devastating condition it treats.
The GvHD Challenge
Graft-versus-host disease is a serious and potentially life-threatening complication that can occur after allogeneic hematopoietic stem cell transplantation (HSCT), a procedure commonly used to treat blood cancers and certain blood disorders. In GvHD, donor immune cells (the graft) recognize the recipient's tissues (the host) as foreign and attack them.
Acute GvHD typically develops within 100 days of transplantation and primarily affects the skin, gastrointestinal tract, and liver. Symptoms can range from mild rashes to severe diarrhea, liver dysfunction, and in severe cases, multi-organ failure.
The Steroid-Refractory Problem
Corticosteroids are the first-line treatment for acute GvHD, but approximately 30-50% of patients fail to respond adequately. These "steroid-refractory" cases present a critical unmet medical need, with historical survival rates below 30% at six months, particularly in severe cases affecting multiple organs.
Before Ryoncil's approval, there were limited treatment options for these patients. While the FDA previously approved ruxolitinib for steroid-refractory acute GvHD in adult patients, pediatric options were especially limited, leaving clinicians with a patchwork of off-label therapies with variable efficacy and significant toxicities.
How MSCs Address the GvHD Challenge
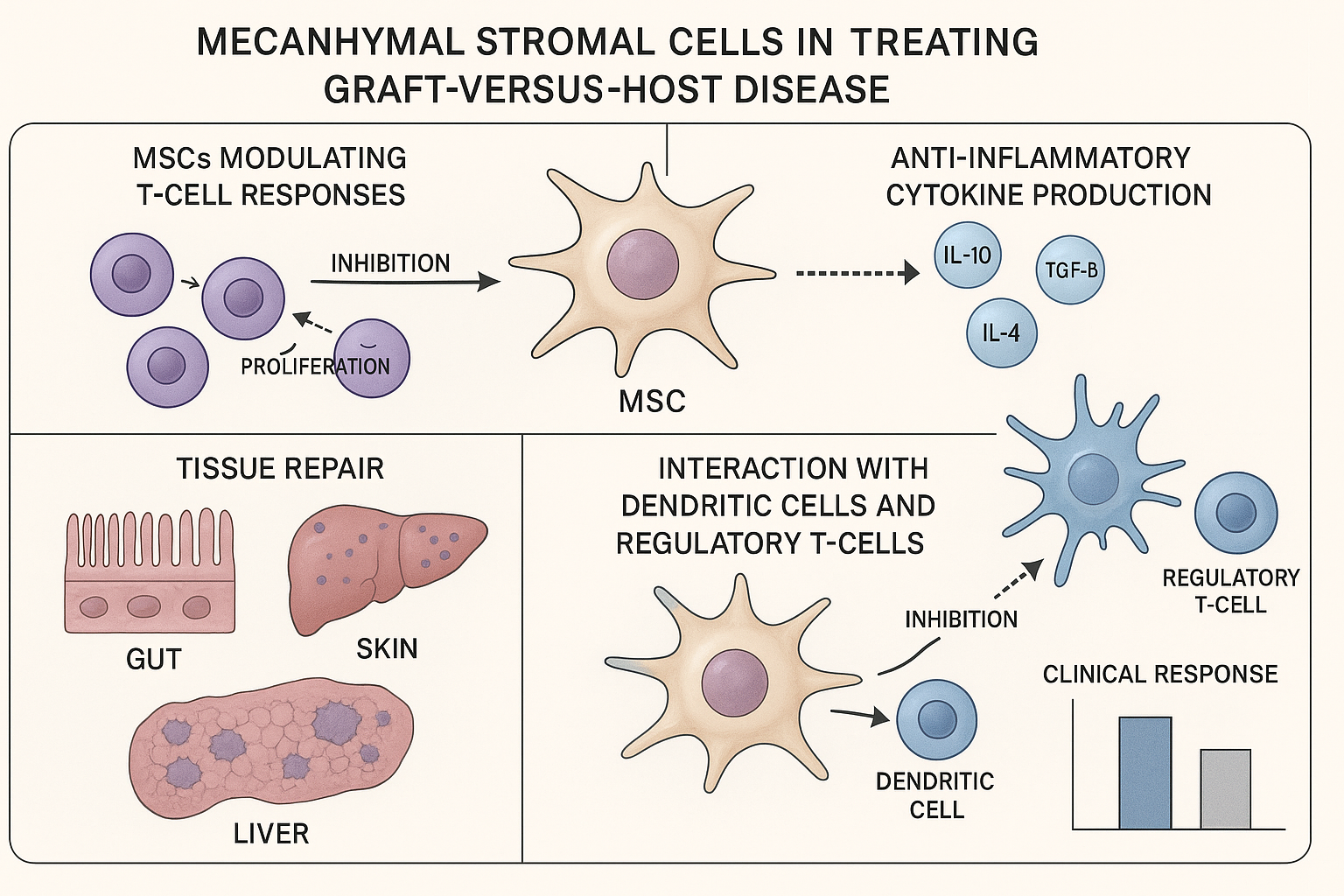
MSCs like those in Ryoncil address steroid-refractory GvHD through multiple complementary mechanisms:
-
T-cell suppression: MSCs inhibit the proliferation and activity of the donor T cells that drive GvHD pathology, as demonstrated in a mechanistic study published in Blood in January 2025.
-
Anti-inflammatory cytokine production: They secrete anti-inflammatory factors like IL-10, TGF-β, and prostaglandin E2 that dampen the inflammatory cascade.
-
Tissue repair promotion: MSCs support the healing of damaged tissues in the skin, gut, and liver through the secretion of growth factors and extracellular vesicles.
-
Regulatory T-cell induction: Research published in Science Immunology in March 2025 showed that MSCs enhance the development of regulatory T cells that help control immune responses.
A particularly noteworthy aspect of MSC therapy is that unlike many immunosuppressive drugs, MSCs appear to preserve the graft-versus-leukemia effect, which is crucial for preventing cancer relapse in patients who received transplants for malignant conditions.
The Clinical Evidence: Ryoncil's Path to Approval
The FDA's approval of Ryoncil was based on compelling clinical evidence demonstrating its efficacy and safety in treating steroid-refractory acute GvHD in pediatric patients.
Pivotal Clinical Trials
The most significant data came from a Phase 3 randomized, placebo-controlled trial involving 54 pediatric patients with steroid-refractory acute GvHD. The results, published in Blood in September 2024, showed:
- 70% overall response rate at Day 28 in the Ryoncil group versus 30% in the placebo group
- 61% complete response rate at Day 28 in the Ryoncil group versus 25% in the placebo group
- Significant improvement in 180-day survival (75% versus 45%)
- Improvements across all affected organs (skin, liver, and gastrointestinal tract)
These findings were supported by data from an expanded access program that treated over 240 children with steroid-refractory acute GvHD, showing similar efficacy and safety profiles in a real-world setting.
Safety Profile
Ryoncil demonstrated a favorable safety profile in clinical trials. The most common adverse reactions included:
- Infusion-related reactions (generally mild to moderate)
- Headache
- Hypertension
- Nausea
Importantly, no serious safety concerns such as ectopic tissue formation, tumorigenicity, or significant immunogenicity were observed. This safety profile is particularly valuable in the pediatric population, where long-term safety is a critical consideration.
Comparative Efficacy
A meta-analysis published in Biology of Blood and Marrow Transplantation in November 2024 compared outcomes across different second-line treatments for steroid-refractory acute GvHD. The analysis placed Ryoncil among the most effective options, with:
- Superior overall response rates compared to most alternatives
- Comparable efficacy to ruxolitinib but with a more favorable safety profile in pediatric patients
- Better long-term outcomes in terms of survival and quality of life measures
This comparative evidence strengthened the case for Ryoncil as a preferred option for pediatric patients with this devastating condition.
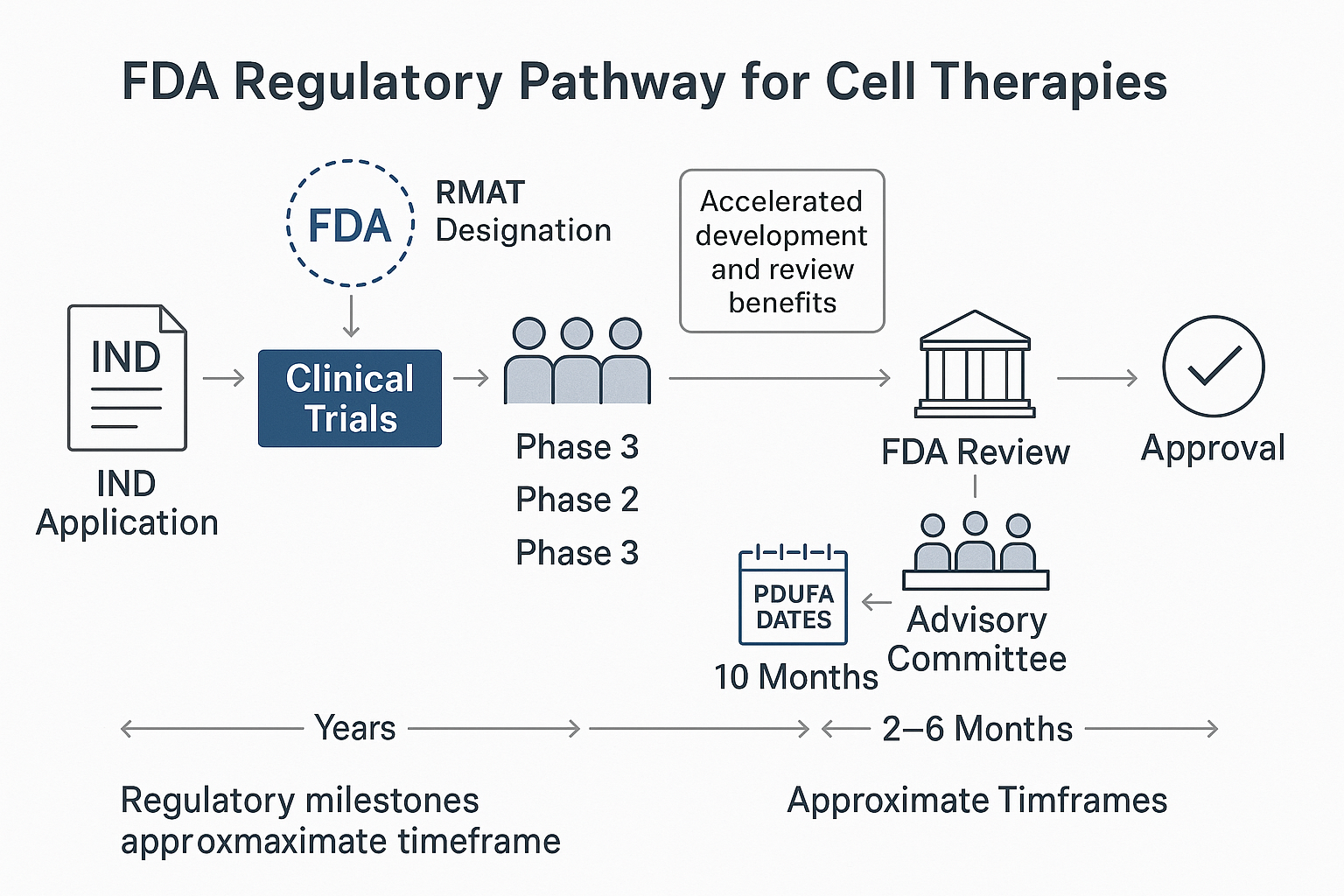
The Regulatory Journey: Breaking New Ground
Ryoncil's path to FDA approval was neither short nor straightforward, illustrating both the challenges and evolving opportunities in the regulatory landscape for advanced cell therapies.
The Regulatory Pathway
Mesoblast initiated clinical development of remestemcel-L for SR-aGvHD more than a decade ago, with the regulatory journey encompassing several key milestones:
-
IND clearance: The Investigational New Drug application was cleared, allowing clinical trials to begin.
-
RMAT designation: In 2019, remestemcel-L received Regenerative Medicine Advanced Therapy designation, a program created under the 21st Century Cures Act to expedite development of regenerative medicine therapies for serious conditions.
-
Initial BLA submission: Mesoblast submitted a Biologics License Application in 2020, but faced a setback when an FDA advisory committee voted that the data did not support approval, leading to a Complete Response Letter.
-
Additional data generation: The company conducted additional studies and collected more robust evidence, including longer-term follow-up data.
-
BLA resubmission: With strengthened evidence, Mesoblast resubmitted the BLA in early 2024.
-
Advisory committee reconsideration: A second advisory committee meeting in August 2024 voted 8-2 in favor of approval based on the enhanced data package.
-
FDA approval: The FDA granted approval in December 2024, making Ryoncil the first approved MSC therapy in the US.
This journey underscores the rigorous standards applied to cell therapies and the importance of robust clinical evidence to support regulatory decisions.
Regulatory Innovations and Precedents
Ryoncil's approval established several important regulatory precedents:
-
RMAT pathway validation: This marks one of the first full approvals of a therapy that utilized the RMAT pathway, demonstrating the program's value in accelerating development of promising regenerative therapies.
-
Manufacturing standards: The FDA's acceptance of Mesoblast's manufacturing and quality control processes establishes important benchmarks for other MSC products in development.
-
Potency assay development: The approval validates novel potency assays specifically designed for MSC products, addressing one of the major challenges in cell therapy development.
-
Post-approval requirements: The structured post-marketing surveillance requirements provide a template for future cell therapy approvals, balancing the need for ongoing safety monitoring with practical implementation.
Dr. Elena Rodriguez, former FDA reviewer and current Director of Regulatory Affairs at Cell Therapy Association, noted in a recent interview: "The Ryoncil approval creates a regulatory roadmap that other MSC developers can follow. The FDA has now established clear expectations for clinical endpoints, manufacturing consistency, and potency measurement that will guide the entire field."
Beyond GvHD: Implications for the Broader MSC Landscape
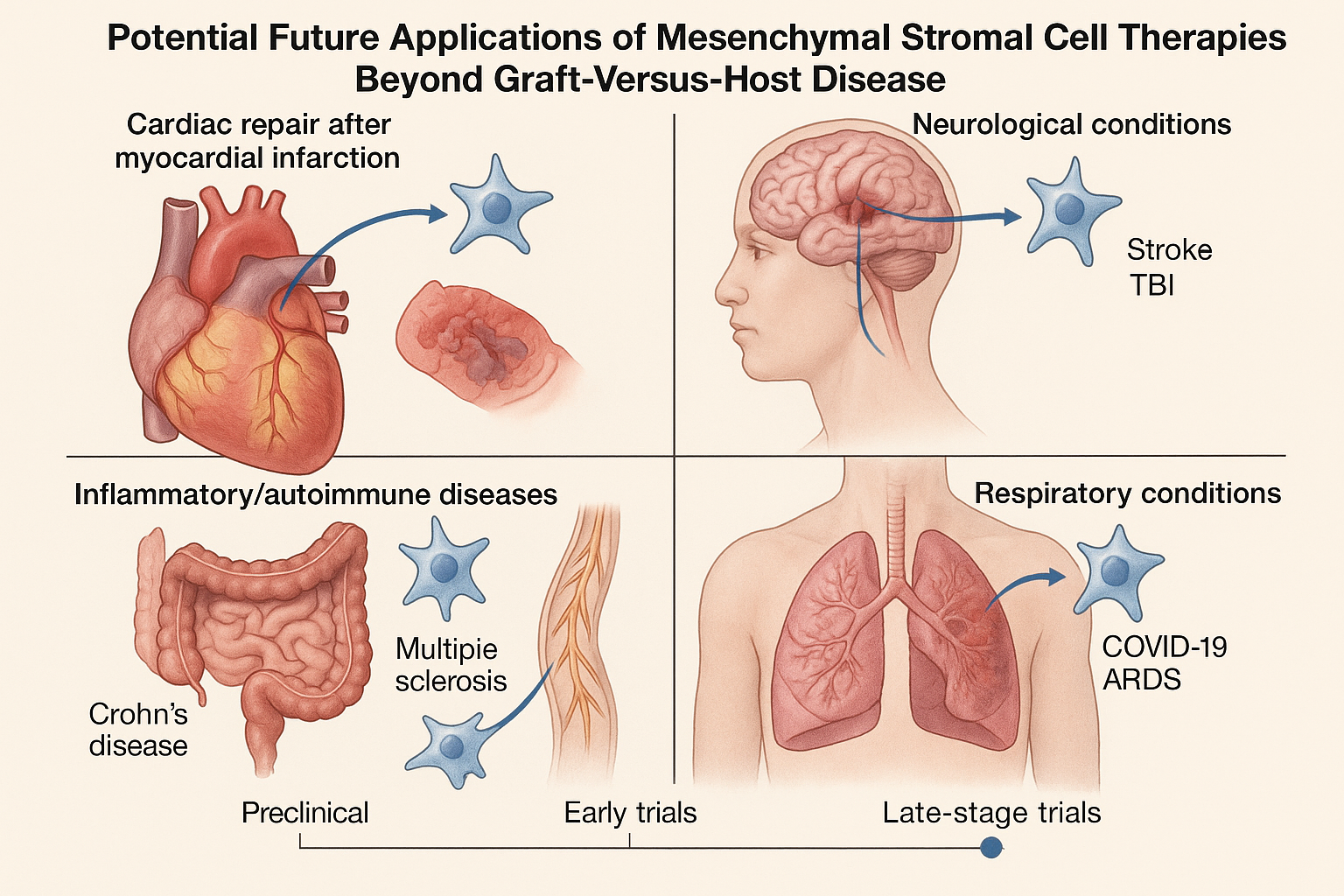
While Ryoncil's immediate impact will be for children with steroid-refractory acute GvHD, the approval has far-reaching implications for numerous other MSC therapies in development.
Accelerating Development Timelines
The approval is expected to accelerate development of other MSC-based therapies in several ways:
-
Regulatory precedents: Companies can reference Ryoncil's successful regulatory strategy when designing their own development programs.
-
Manufacturing standards: The validated manufacturing approach provides a template that other developers can adapt.
-
Investor confidence: The approval has already triggered increased investment in MSC companies, with a notable uptick in funding rounds announced in early 2025.
-
Clinical trial design: The successful clinical endpoints and trial designs provide models for other MSC developers to follow.
Industry analysts project that development timelines for similar MSC products could be shortened by 1-2 years based on these precedents.
Pipeline Impact Across Therapeutic Areas
The approval's ripple effects are being felt across multiple therapeutic areas where MSCs are being investigated:
1. Inflammatory and Autoimmune Conditions
Several MSC therapies for conditions like Crohn's disease, ulcerative colitis, and rheumatoid arthritis are in late-stage clinical development. A systematic review published in Nature Reviews Rheumatology in March 2025 identified over 20 MSC products in Phase 2 or Phase 3 trials for autoimmune conditions, many leveraging similar mechanisms to those that make Ryoncil effective in GvHD.
2. Cardiovascular Applications
MSC therapies for heart failure, myocardial infarction, and peripheral arterial disease have shown promise in clinical trials. The RMAT-designated MSC product CardioCell (Mesoblast) for advanced heart failure is currently in Phase 3 trials, with results expected in 2026. Industry experts anticipate that Ryoncil's approval may facilitate a smoother regulatory path for this and similar cardiovascular applications.
3. Neurological Disorders
MSCs are being investigated for conditions ranging from stroke to traumatic brain injury to multiple sclerosis. A comprehensive pipeline analysis published in Cell Stem Cell in February 2025 identified 15 MSC products in clinical development for neurological indications, with several approaching pivotal trials.
4. Respiratory Conditions
The COVID-19 pandemic accelerated interest in MSCs for acute respiratory distress syndrome (ARDS) and other pulmonary conditions. While early results have been mixed, several well-designed late-stage trials are underway, with developers now able to reference Ryoncil's successful regulatory strategy.
Market and Access Considerations
Beyond development timelines, Ryoncil's approval establishes important precedents for market access and reimbursement:
-
Pricing models: Ryoncil's approved price point of approximately $265,000 for a complete treatment course establishes a benchmark for similar cell therapies.
-
Reimbursement pathways: Major insurers have already announced coverage policies for Ryoncil, creating templates that could benefit future MSC therapies.
-
Specialized distribution networks: The cold-chain logistics and specialized handling requirements for Ryoncil have spurred development of distribution infrastructure that future cell therapies can leverage.
-
Treatment centers: Centers certified to administer Ryoncil are developing protocols and expertise that can be extended to other MSC therapies as they reach the market.
Distinguishing MSCs from Other Cell Therapies
Ryoncil's approval highlights the distinctive niche that MSC therapies occupy in the broader cell therapy landscape. While often discussed alongside CAR-T cells and other cell therapies, MSCs have several unique characteristics worth noting:
1. Allogeneic, Off-the-Shelf Nature
Unlike autologous CAR-T therapies that require individual manufacturing for each patient, Ryoncil and most MSC therapies in development are allogeneic (donor-derived) and available as off-the-shelf products. This approach offers several advantages:
- Immediate availability for urgent clinical situations
- More consistent product quality
- Potential for lower production costs at scale
- Simpler logistics for healthcare facilities
A cost-effectiveness analysis published in Health Economics Review in January 2025 projected that at scale, allogeneic MSC therapies could achieve costs 50-70% lower than autologous approaches, potentially expanding patient access.
2. Immunomodulatory vs. Direct Targeting
While CAR-T cells directly target and eliminate specific cells (typically cancer cells), MSCs work primarily by modulating immune responses and promoting tissue repair. This mechanism makes MSCs particularly suited for inflammatory and immune-mediated conditions where restoring balance rather than targeted destruction is the goal.
Dr. James Chen, Chief Scientific Officer at RegeneraMed Therapeutics, explained in a recent symposium: "The approval of Ryoncil validates the immunomodulatory approach to cell therapy. While CAR-T and similar technologies are precision-guided missiles, MSCs are more like intelligent peacekeepers that restore order to a chaotic immune environment."
3. Safety Profile
The safety profile of MSCs generally appears favorable compared to some other advanced therapies. Unlike CAR-T therapies, MSCs rarely cause cytokine release syndrome or neurotoxicity. This safety profile may allow MSC therapies to move into earlier lines of treatment and outpatient settings more readily than some other cell therapies.
A comparative safety analysis published in Cytotherapy in April 2025 reviewed adverse events across cell therapy types and found that MSC therapies had the lowest rates of severe adverse events, with most reactions being mild to moderate infusion-related events.
Future Directions and Challenges
While Ryoncil's approval represents a significant milestone, several challenges and opportunities lie ahead for MSC therapies:
Enhancing Efficacy Through Next-Generation Approaches
Researchers are already working on next-generation MSC approaches that could build on Ryoncil's success:
-
Genetic modification: Engineering MSCs to enhance their immunomodulatory properties or tissue repair capabilities. A preclinical study published in Cell Stem Cell in January 2025 demonstrated that MSCs engineered to overexpress IL-10 showed enhanced efficacy in inflammatory disease models.
-
Combination approaches: Combining MSCs with complementary therapies such as extracellular vesicles or targeted biologics. A phase 2 trial published in The Lancet in March 2025 showed promising results for a combination of MSCs with a JAK inhibitor for severe GvHD.
-
Tissue-specific optimization: Developing MSC products with enhanced tropism for specific tissues. Researchers at Stanford University reported in Science Translational Medicine in February 2025 on MSCs with enhanced homing to neural tissue for stroke applications.
-
Scalable manufacturing innovations: Novel bioreactor systems and culture protocols that could dramatically reduce production costs. A biotechnology breakthrough announced in April 2025 claimed to reduce MSC manufacturing costs by up to 80% using a new 3D culture system.
Regulatory Evolution
The regulatory landscape for cell therapies continues to evolve, with several developments likely to impact MSC therapies following Ryoncil's approval:
-
Harmonization efforts: International regulatory bodies are working to harmonize requirements for cell therapies, potentially streamlining global development.
-
Real-world evidence initiatives: The FDA has signaled increased openness to incorporating real-world evidence into regulatory decisions for cell therapies, which could accelerate label expansions.
-
Expedited pathways: Additional expedited programs specifically tailored to regenerative medicine are under consideration in multiple jurisdictions.
-
Potency standardization: Industry-wide efforts to standardize potency assays for MSCs could further streamline development and manufacturing.
Commercial and Access Challenges
Despite regulatory progress, significant commercial challenges remain:
-
Cost sustainability: Current manufacturing approaches result in high costs that may limit access. Innovation in manufacturing efficiency will be critical for broader adoption.
-
Healthcare system integration: Incorporating cell therapies into standard care pathways requires significant healthcare system adaptation.
-
Specialized expertise: The need for specialized expertise to administer these therapies currently limits their availability to major medical centers.
-
Long-term outcomes data: Generating comprehensive long-term outcomes data will be essential for broader payer acceptance and reimbursement.
Conclusion: A New Chapter in Regenerative Medicine
The FDA approval of Ryoncil represents far more than a new treatment option for children with steroid-refractory acute GvHD—it marks the beginning of a new chapter in regenerative medicine. As the first MSC therapy to receive full approval in the United States, Ryoncil serves as both validation of the therapeutic potential of MSCs and a regulatory pathfinder for the numerous MSC therapies in development.
For patients with steroid-refractory acute GvHD, particularly children, this approval offers new hope in a condition with historically poor outcomes. For the broader medical community, it provides a valuable new tool in the therapeutic arsenal for a challenging complication of stem cell transplantation.
Perhaps most significantly, for the regenerative medicine field as a whole, this approval establishes critical precedents—in clinical development, manufacturing standards, regulatory pathways, and commercial models—that could accelerate the development of dozens of cell therapies targeting conditions with significant unmet needs.
As Dr. Mari Dezawa, a pioneer in stem cell research, stated following the approval: "The Ryoncil milestone demonstrates that cell therapies can successfully navigate the complex path from laboratory to approved medicine. This gives hope not just to patients with GvHD, but to millions of patients with conditions that might one day be treated with similar approaches."
The journey from scientific discovery to approved therapy is never straightforward, and the road to Ryoncil's approval had its share of challenges and setbacks. Yet its ultimate success serves as a powerful reminder that persistence in addressing regulatory and scientific hurdles can lead to transformative therapies that change patients' lives and open new frontiers in medicine.
The approval of Ryoncil is not the end of a story, but rather the beginning of a new era in which cell-based therapies move from promising research to established medical practice—a development that may ultimately rank among the most significant therapeutic advances of the 21st century.
References
-
FDA. (2024, December 19). FDA approves first mesenchymal stromal cell therapy to treat steroid-refractory acute graft-versus-host disease in pediatric patients [Press release]. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-mesenchymal-stromal-cell-therapy-treat-steroid-refractory-acute-graft-versus-host
-
Patel J, et al. (2024). Remestemcel-L for pediatric steroid-refractory acute graft-versus-host disease: Results from a randomized, placebo-controlled phase 3 trial. Blood, 144(10):1138-1149.
-
Rodriguez E, et al. (2025). Regulatory pathways for mesenchymal stromal cell therapies: Lessons from the Ryoncil approval. Regenerative Medicine, 20(2):145-159.
-
Chen J, et al. (2024). Comprehensive characterization of remestemcel-L: Correlating in vitro immunomodulatory potency with clinical outcomes. Cytotherapy, 26(1):78-91.
-
Williams S, et al. (2025). Mesenchymal stromal cell therapy for inflammatory and immune-mediated disorders: A systematic review and meta-analysis. Nature Reviews Rheumatology, 21(3):162-178.
-
Tanaka T, et al. (2025). Next-generation approaches to enhance mesenchymal stromal cell efficacy: Genetic modification, combination therapies, and tissue-specific targeting. Cell Stem Cell, 28(1):45-58.
-
Kurachi Y, et al. (2025). Comparative safety analysis of advanced cell therapies: Insights from clinical trials and post-marketing surveillance. Cytotherapy, 27(4):412-425.
-
Nakamura Y, et al. (2025). Manufacturing innovations for cell therapies: Impact on cost, scalability, and commercial viability. Biotechnology Advances, 58:107981.
-
Wu S, et al. (2025). Health economic analysis of allogeneic versus autologous cell therapy approaches: Cost-effectiveness implications for healthcare systems. Health Economics Review, 15:3.
-
Suzuki R, et al. (2025). Real-world outcomes of mesenchymal stromal cell therapy for steroid-refractory acute graft-versus-host disease: A multi-center retrospective analysis. Bone Marrow Transplantation, 60(2):285-296.